Mmp1 processing of the PDF neuropeptide regulates circadian structural plasticity of pacemaker neurons
- PMID: 25356918
- PMCID: PMC4214601
- DOI: 10.1371/journal.pgen.1004700
Mmp1 processing of the PDF neuropeptide regulates circadian structural plasticity of pacemaker neurons
Abstract
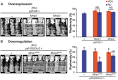
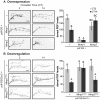
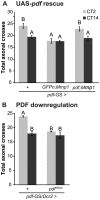
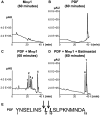
In the Drosophila brain, the neuropeptide PIGMENT DISPERSING FACTOR (PDF) is expressed in the small and large Lateral ventral neurons (LNvs) and regulates circadian locomotor behavior. Interestingly, PDF immunoreactivity at the dorsal terminals changes across the day as synaptic contacts do as a result of a remarkable remodeling of sLNv projections. Despite the relevance of this phenomenon to circuit plasticity and behavior, the underlying mechanisms remain poorly understood. In this work we provide evidence that PDF along with matrix metalloproteinases (Mmp1 and 2) are key in the control of circadian structural remodeling. Adult-specific downregulation of PDF levels per se hampers circadian axonal remodeling, as it does altering Mmp1 or Mmp2 levels within PDF neurons post-developmentally. However, only Mmp1 affects PDF immunoreactivity at the dorsal terminals and exerts a clear effect on overt behavior. In vitro analysis demonstrated that PDF is hydrolyzed by Mmp1, thereby suggesting that Mmp1 could directly terminate its biological activity. These data demonstrate that Mmp1 modulates PDF processing, which leads to daily structural remodeling and circadian behavior.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Coupling Neuropeptide Levels to Structural Plasticity in Drosophila Clock Neurons.Curr Biol. 2020 Aug 17;30(16):3154-3166.e4. doi: 10.1016/j.cub.2020.06.009. Epub 2020 Jul 2. Curr Biol. 2020. PMID: 32619484
-
The Drosophila Receptor Protein Tyrosine Phosphatase LAR Is Required for Development of Circadian Pacemaker Neuron Processes That Support Rhythmic Activity in Constant Darkness But Not during Light/Dark Cycles.J Neurosci. 2016 Mar 30;36(13):3860-70. doi: 10.1523/JNEUROSCI.4523-15.2016. J Neurosci. 2016. PMID: 27030770 Free PMC article.
-
The neuropeptide PDF acts directly on evening pacemaker neurons to regulate multiple features of circadian behavior.PLoS Biol. 2009 Jul;7(7):e1000154. doi: 10.1371/journal.pbio.1000154. Epub 2009 Jul 21. PLoS Biol. 2009. PMID: 19621061 Free PMC article.
-
Circadian pathway: the other shoe drops.Curr Biol. 2005 Dec 20;15(24):R987-9. doi: 10.1016/j.cub.2005.11.053. Curr Biol. 2005. PMID: 16360675 Review.
-
The circadian system: plasticity at many levels.Neuroscience. 2013 Sep 5;247:280-93. doi: 10.1016/j.neuroscience.2013.05.036. Epub 2013 May 29. Neuroscience. 2013. PMID: 23727010 Review.
Cited by
-
The Drosophila circadian clock gene cycle controls the development of clock neurons.PLoS Genet. 2024 Oct 21;20(10):e1011441. doi: 10.1371/journal.pgen.1011441. eCollection 2024 Oct. PLoS Genet. 2024. PMID: 39432537 Free PMC article.
-
Regulation of PDF receptor signaling controlling daily locomotor rhythms in Drosophila.PLoS Genet. 2022 May 23;18(5):e1010013. doi: 10.1371/journal.pgen.1010013. eCollection 2022 May. PLoS Genet. 2022. PMID: 35605015 Free PMC article.
-
A novel Drosophila injury model reveals severed axons are cleared through a Draper/MMP-1 signaling cascade.Elife. 2017 Aug 21;6:e23611. doi: 10.7554/eLife.23611. Elife. 2017. PMID: 28825401 Free PMC article.
-
High-Frequency Neuronal Bursting is Essential for Circadian and Sleep Behaviors in Drosophila.J Neurosci. 2021 Jan 27;41(4):689-710. doi: 10.1523/JNEUROSCI.2322-20.2020. Epub 2020 Dec 1. J Neurosci. 2021. PMID: 33262246 Free PMC article.
-
Emerging roles for microRNA in the regulation of Drosophila circadian clock.BMC Neurosci. 2018 Jan 16;19(1):1. doi: 10.1186/s12868-018-0401-8. BMC Neurosci. 2018. PMID: 29338692 Free PMC article. Review.
References
-
- Ozkaya O, Rosato E (2012) The circadian clock of the fly: a neurogenetics journey through time. Adv Genet 77: 79–123. - PubMed
-
- Frenkel L, Ceriani MF (2011) Circadian plasticity: from structure to behavior. Int Rev Neurobiol 99: 107–138. - PubMed
-
- Helfrich-Forster C, Shafer OT, Wulbeck C, Grieshaber E, Rieger D, et al. (2007) Development and morphology of the clock-gene-expressing lateral neurons of Drosophila melanogaster. J Comp Neurol 500: 47–70. - PubMed
-
- Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH (1999) A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell 99: 791–802. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

