Biochemical, mechanistic, and spectroscopic characterization of metallo-β-lactamase VIM-2
- PMID: 25356958
- PMCID: PMC4245990
- DOI: 10.1021/bi500916y
Biochemical, mechanistic, and spectroscopic characterization of metallo-β-lactamase VIM-2
Abstract
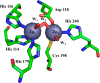
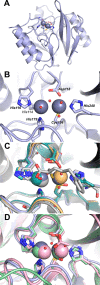
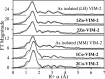
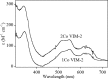
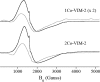
This study examines metal binding to metallo-β-lactamase VIM-2, demonstrating the first successful preparation of a Co(II)-substituted VIM-2 analogue. Spectroscopic studies of the half- and fully metal loaded enzymes show that both Zn(II) and Co(II) bind cooperatively, where the major species present, regardless of stoichiometry, are apo- and di-Zn (or di-Co) enzymes. We determined the di-Zn VIM-2 structure to a resolution of 1.55 Å, and this structure supports results from spectroscopic studies. Kinetics, both steady-state and pre-steady-state, show that VIM-2 utilizes a mechanism that proceeds through a very short-lived anionic intermediate when chromacef is used as the substrate. Comparison with other B1 enzymes shows that those that bind Zn(II) cooperatively are better poised to protonate the intermediate on its formation, compared to those that bind Zn(II) non-cooperatively, which uniformly build up substantial amounts of the intermediate.
Figures
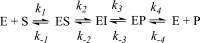

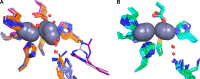
References
-
- CDC (2013) Antibiotic resistance threats in the United States, http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013.... - PubMed
-
- Bush K. (2013) The ABCD’s of β-lactamase nomenclature. J. Infect. Chemother. 19, 549–559. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

