Antiangiogenic variant of TSP-1 targets tumor cells in glioblastomas
- PMID: 25358253
- PMCID: PMC4445617
- DOI: 10.1038/mt.2014.214
Antiangiogenic variant of TSP-1 targets tumor cells in glioblastomas
Expression of concern in
-
Antiangiogenic Variant of TSP-1 Targets Tumor Cells in Glioblastomas.Mol Ther. 2024 May 1;32(5):1595. doi: 10.1016/j.ymthe.2024.04.022. Epub 2024 Apr 18. Mol Ther. 2024. PMID: 38702134 Free PMC article. No abstract available.
Abstract
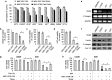
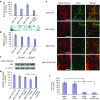
Three type-1 repeat (3TSR) domain of thrombospondin-1 is known to have anti-angiogenic effects by targeting tumor-associated endothelial cells, but its effect on tumor cells is unknown. This study explored the potential of 3TSR to target glioblastoma (GBM) cells in vitro and in vivo. We show that 3TSR upregulates death receptor (DR) 4/5 expression in a CD36-dependent manner and primes resistant GBMs to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced caspase-8/3/7 mediated apoptosis. We engineered human mesenchymal stem cells (MSC) for on-site delivery of 3TSR and a potent and secretable variant of TRAIL (S-TRAIL) in an effort to simultaneously target tumor cells and associated endothelial cells and circumvent issues of systemic delivery of drugs across the blood-brain barrier. We show that MSC-3TSR/S-TRAIL inhibits tumor growth in an expanded spectrum of GBMs. In vivo, a single administration of MSC-3TSR/S-TRAIL significantly targets both tumor cells and vascular component of GBMs, inhibits tumor progression, and extends survival of mice bearing highly vascularized GBM. The ability of 3TSR/S-TRAIL to simultaneously act on tumor cells and tumor-associated endothelial cells offers a great potential to target a broad spectrum of cancers and translate 3TSR/TRAIL therapies into clinics.
Figures


References
-
- Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610–622. - PubMed
-
- Plate KH, Mennel HD. Vascular morphology and angiogenesis in glial tumors. Exp Toxicol Pathol. 1995;47:89–94. - PubMed
-
- Rampling R, Cruickshank G, Lewis AD, Fitzsimmons SA, Workman P. Direct measurement of pO2 distribution and bioreductive enzymes in human malignant brain tumors. Int J Radiat Oncol Biol Phys. 1994;29:427–431. - PubMed
-
- Valk PE, Mathis CA, Prados MD, Gilbert JC, Budinger TF. Hypoxia in human gliomas: demonstration by PET with fluorine-18-fluoromisonidazole. J Nucl Med. 1992;33:2133–2137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

