Acetylation preserves retinal ganglion cell structure and function in a chronic model of ocular hypertension
- PMID: 25358731
- PMCID: PMC4240722
- DOI: 10.1167/iovs.14-14792
Acetylation preserves retinal ganglion cell structure and function in a chronic model of ocular hypertension
Abstract
Purpose: The current studies investigate if the histone deacetylase (HDAC) inhibitor, valproic acid (VPA), can limit retinal ganglion cell (RGC) degeneration in an ocular-hypertensive rat model.
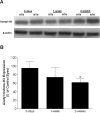
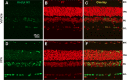
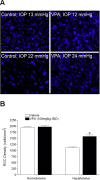
Methods: Intraocular pressure (IOP) was elevated unilaterally in Brown Norway rats by hypertonic saline injection. Rats received either vehicle or VPA (100 mg/kg) treatment for 28 days. Retinal ganglion cell function and number were assessed by pattern electroretinogram (pERG) and retrograde FluoroGold labeling. Western blotting and a fluorescence assay were used for determination of histone H3 acetylation and HDAC activity, respectively, at 3-day, 1-week, and 2-week time points.
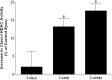
Results: Hypertonic saline injections increased IOPs by 7 to 14 mm Hg. In vehicle-treated animals, ocular hypertension resulted in a 29.1% and 39.4% decrease in pERG amplitudes at 2 and 4 weeks, respectively, and a 42.9% decrease in mean RGC density at 4 weeks. In comparison, VPA treatment yielded significant amplitude preservation at 2 and 4 weeks and showed significant RGC density preservation at 4 weeks. No significant difference in RGC densities or IOPs was measured between control eyes of vehicle- and VPA-treated rats. In ocular-hypertensive eyes, class I HDAC activity was significantly elevated within 1 week (13.3 ± 2.2%) and histone H3 acetylation was significantly reduced within 2 weeks following the induction of ocular hypertension.
Conclusions: Increase in HDAC activity is a relatively early retinal event induced by elevated IOP, and suppressing HDAC activity can protect RGCs from ocular-hypertensive stress. Together these data provide a basis for developing HDAC inhibitors for the treatment of optic neuropathies.
Keywords: acetylation; neuroprotection; ocular hypertension; retina.
Copyright 2014 The Association for Research in Vision and Ophthalmology, Inc.
Figures



References
-
- Naskar R, Wissing M, Thanos S. Detection of early neuron degeneration and accompanying microglial responses in the retina of a rat model of glaucoma. Invest Ophthalmol Vis Sci. 2002; 43: 2962–2968. - PubMed
-
- Quigley HA. Glaucoma. Lancet. 2011; 377: 1367–1377. - PubMed
-
- Biermann J, Grieshaber P, Goebel U, et al. Valproic acid-mediated neuroprotection and regeneration in injured retinal ganglion cells. Invest Ophthalmol Vis Sci. 2010; 51: 526–534. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

