Cilostazol for intermittent claudication
- PMID: 25358850
- PMCID: PMC7173701
- DOI: 10.1002/14651858.CD003748.pub4
Cilostazol for intermittent claudication
Update in
-
Cilostazol for intermittent claudication.Cochrane Database Syst Rev. 2021 Jun 30;6(6):CD003748. doi: 10.1002/14651858.CD003748.pub5. Cochrane Database Syst Rev. 2021. PMID: 34192807 Free PMC article.
Abstract
Background: Peripheral arterial disease (PAD) affects between 4% and 12% of people aged 55 to 70 years, and 20% of people over 70 years. A common complaint is intermittent claudication, characterised by pain in the legs or buttocks that occurs with exercise and which subsides with rest. Compared with age-matched controls, people with intermittent claudication have a three- to six-fold increase in cardiovascular mortality. Symptoms of intermittent claudication, walking distance, and quality of life can be improved by risk factor modification, smoking cessation, and a structured exercise programme. Antiplatelet treatment is beneficial in patients with intermittent claudication for the reduction of vascular events but has not previously been shown to influence claudication distance. This is an update of a review first published in 2007.
Objectives: To determine the effect of cilostazol (an antiplatelet treatment) on improving initial and absolute claudication distances, and in reducing mortality and vascular events in patients with stable intermittent claudication.
Search methods: For this update, the Cochrane Peripheral Vascular Diseases Group Trials Search Co-ordinator searched the Specialised Register (last searched October 2013) and CENTRAL (2013, Issue 9).
Selection criteria: Double-blind, randomised controlled trials (RCTs) of cilostazol versus placebo, or versus other antiplatelet agents in patients with stable intermittent claudication.
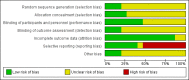
Data collection and analysis: Two authors independently assessed trials for selection and independently extracted data. Disagreements were resolved by discussion. We performed the meta-analysis as a fixed-effect model with weighted mean differences (WMDs) and 95% confidence intervals (CIs) for continuous data, and odds ratios (ORs) with 95% CIs for dichotomous data.
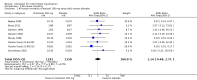
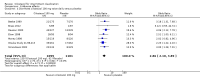
Main results: We included fifteen double-blind, RCTs comparing cilostazol with placebo, or medications currently known to increase walking distance e.g. pentoxifylline. There were a total of 3718 randomised participants with treatment durations ranging from six to 26 weeks. All participants had intermittent claudication secondary to PAD. Comparisons included cilostazol twice daily, with dosages of 50 mg, 100 mg and 150 mg compared with placebo, and cilostazol 100 mg, twice daily, compared with pentoxifylline 400 mg, three times daily. The methodological quality of the trials was generally low, with the majority being at an unclear risk for selection bias, performance bias, detection bias and other bias. Attrition bias was generally low, but reporting bias was high or unclear in the majority of the studies. For eight studies data were compatible for comparison by meta-analysis, but data for seven studies were too heterogenous to be pooled. For the studies included in the meta-analysis, for initial claudication distance (ICD - the distance walked on a treadmill before the onset of calf pain) there was an improvement in the cilostazol group for the 100 mg and 50 mg twice daily, compared with placebo (WMD 31.41 metres, 95% CI 22.38 to 40.45 metres; P < 0.00001) and WMD 19.89 metres, 95% CI 9.44 to 30.34 metres; P = 0.0002), respectively. ICD was improved in the cilostazol group for the comparison of cilostazol 150 mg versus placebo and cilostazol 100 mg versus pentoxifylline, but only single studies were used for these analyses. Absolute claudication distance (ACD - the maximum distance walked on a treadmill) was significantly increased in participants taking cilostazol 100 mg and 50 mg twice daily, compared with placebo (WMD 43.12 metres, 95% CI 18.28 to 67.96 metres; P = 0.0007) and WMD 32.00 metres, 95% CI 14.17 to 49.83 metres; P = 0.0004), respectively. As with ICD, ACD was increased in participants taking cilostazol 150 mg versus placebo, but with only one study an association cannot be clearly determined. Two studies comparing cilostazol to pentoxifylline had opposing findings, resulting in an imprecise CI (WMD 13.42 metres (95% CI -43.51 to 70.35 metres; P = 0.64). Ankle brachial index (ABI) was lowered in the cilostazol 100 mg group compared with placebo (WMD 0.06, 95% CI 0.04 to 0.08; P < 0.00001). The single study evaluating ABI for the comparison of cilostazol versus pentoxifylline found no change in ABI.There was no association between treatment type and all-cause mortality for any of the treatment comparisons, but there were very few events, and therefore larger, adequately powered studies will be needed to assess if there is a relationship. Only one study evaluated individual cardiovascular events, and from this study there is no clear evidence of a difference between any of the treatment groups and risk of myocardial infarction or stroke. We evaluated adverse side effects, and in general cilostazol was associated with a higher odds of headache, diarrhoea, abnormal stool, dizziness and palpitations. We only reported quality of life measures descriptively as there was insufficient statistical detail within the studies to combine the results, although there was a possible indication in improvement of quality of life in the cilostazol treatment groups.
Authors' conclusions: Cilostazol has been shown to be of benefit in improving walking distance in people with intermittent claudication secondary to PAD. Although there is an increase in adverse side effects, they are generally mild and treatable. There is currently insufficient data on whether taking cilostazol results in a reduction of all-cause mortality and cardiovascular events or an improvement in quality of life. Future research into the effect of cilostazol on intermittent claudication should carefully consider comparability, sample size and homogeneity when designing a study.
Conflict of interest statement
RB: none known. MS: none known. MC: Otsuka Ltd (producer of Cilostazol) made a donation (Approx. GBP 6000) to a research fund held by Newcastle University in 2003. This fund was used to support research in platelet studies (used to purchase chemicals e.g. antibodies) required for platelet activation laboratory research. The work was submitted as part of an MD thesis in 2006. These studies were not used in this Cochrane review. PR: none known DPM: Attended meetings and advisory boards and given some lectures sponsored by Merck, Sharp and Dohme (MSD) and Genzyme. I am editor‐in‐chief of several journals hence some royalties are paid to me (Bentham publications, SAGE publications and Informa publications). GS: none known.
Figures


































Update of
-
Cilostazol for peripheral arterial disease.Cochrane Database Syst Rev. 2008 Jan 23;(1):CD003748. doi: 10.1002/14651858.CD003748.pub3. Cochrane Database Syst Rev. 2008. Update in: Cochrane Database Syst Rev. 2014 Oct 31;(10):CD003748. doi: 10.1002/14651858.CD003748.pub4. PMID: 18254032 Updated.
References
References to studies included in this review
Beebe 1999 {published data only}
-
- Beebe HG, Dawson DL, Cutler BS, Herd JA, Strandness DE Jr, Bortey EB, et al. A new pharmacological treatment for intermittent claudication: results of a randomized, multicenter trial. Archives of Internal Medicine 1999;159(17):2041‐50. - PubMed
-
- Otsuka America. Pletal (Cilostazol) Tablets. Study 21‐92‐202. www.accessdata.fda.gov/drugsatfda_docs/nda/99/20863.cfm (accessed May 2014).
Brass 2012 {published data only}
-
- Brass EP, Cooper LT, Morgan RE, Hiatt WR. A phase II dose‐ranging study of the phosphodiesterase inhibitor K‐134 in patients with peripheral artery disease and claudication. Journal of Vascular Surgery 2012;55(2):381‐9. - PubMed
-
- NCT00783081. Safety and efficacy of K‐134 for the treatment of intermittent claudication. www.clinicaltrials.gov/ct2/show/NCT00783081 (accessed May 2014).
Dawson 1998 {published data only}
-
- Dawson DL, Cutler BS, Meissner MH, Strandness DE Jr. Cilostazol has beneficial effects in treatment of intermittent claudication: results from a multicenter, randomized, prospective, double‐blind trial. Circulation 1998;98(7):678‐86. - PubMed
-
- Otsuka America. Pletal (Cilostazol) Tablets. Study 21‐90‐201. www.accessdata.fda.gov/drugsatfda_docs/nda/99/20863.cfm (accessed May 2014).
Dawson 2000 {published data only}
-
- Dawson DL, Cutler BS, Hiatt WR, Hobson RW 2nd, Martin JD, Bortey EB, et al. A comparison of cilostazol and pentoxifylline for treating intermittent claudication. American Journal of Medicine 2000;109(7):523‐30. - PubMed
-
- Otsuka America. Pletal (Cilostazol) Tablets. Study 21‐96‐202. www.accessdata.fda.gov/drugsatfda_docs/nda/99/20863.cfm (accessed May 2014).
de Albuquerque 2008 {published data only}
-
- Albuquerque RM, Virgini‐Magalhães CE, Lencastre SF, Bottino DA, Bouskela E. Effects of cilostazol and pentoxifylline on forearm reactive hyperemia response, lipid profile, oxidative stress, and inflammatory markers in patients with intermittent claudication. Angiology 2008;59(5):549‐58. - PubMed
Elam 1998 {published data only}
-
- Elam MB, Heckman J, Crouse JR, Hunninghake DB, Herd JA, Davidson M, et al. Effect of the novel antiplatelet agent cilostazol on plasma lipoproteins in patients with intermittent claudication. Arteriosclerosis, Thrombosis, and Vascular Biology 1998;18(12):1942‐7. - PubMed
-
- Otsuka America. Pletal (Cilostazol) Tablets. Study 21‐93‐201. www.accessdata.fda.gov/drugsatfda_docs/nda/99/20863.cfm (accessed May 2014).
Money 1998 {published data only}
-
- Money SR, Herd JA, Isaacsohn JL, Davidson M, Cutler B, Heckman J, et al. Effect of cilostazol on walking distances in patients with intermittent claudication caused by peripheral vascular disease. Journal of Vascular Surgery 1998;27(2):267‐74. - PubMed
-
- Otsuka America. Pletal (Cilostazol) Tablets. Study 21‐94‐203. www.accessdata.fda.gov/drugsatfda_docs/nda/99/20863.cfm (accessed May 2014).
O'Donnell 2009 {published data only}
-
- O'Donnell M, Badger SA, Sharif MA, Young IS, Lau LL, Lee B, et al. Randomised controlled trial assessing the effects of cilostazol on exercise‐induced ischaemia‐reperfusion in patients with peripheral arterial disease. Proceedings of the European Society for Vascular Surgery Annual Meeting; 2008 Sep 4‐7; Nice, France. 2008:43.
-
- O'Donnell ME, Badger SA, Makar RR, McEneny J, Young IS, Lau LL, et al. The effects of cilostazol on the attenuation of inflammatory response in patients with peripheral arterial disease. The Vascular Society of Great Britain & Ireland Yearbook 2007:49.
-
- O'Donnell ME, Badger SA, Makar RR, Young IS, Lau LL, Lee B, et al. The effects of cilostazol in diabetic patients. The Vascular Society of Great Britain & Ireland Yearbook 2007:82.
-
- O'Donnell ME, Badger SA, Sharif MA, Makar RR, McEneny J, Young IS, et al. The effects of cilostazol on exercise‐induced ischaemia‐reperfusion injury in patients with peripheral arterial disease. European Journal of Vascular and Endovascular Surgery 2009;37(3):326‐35. - PubMed
-
- O'Donnell ME, Badger SA, Sharif MA, Makar RR, Young IS, Lee B, et al. The effects of cilostazol on peripheral neuropathy in diabetic patients with peripheral arterial disease. Angiology 2008;59(6):695‐704. - PubMed
Otsuka Study 21‐86‐101 {unpublished data only}
-
- Otsuka America. Pletal (Cilostazol) Tablets. Study 21‐86‐101. www.accessdata.fda.gov/drugsatfda_docs/nda/99/20863.cfm (accessed May 2014).
Otsuka Study 21‐86‐103 {unpublished data only}
-
- Otsuka America. Pletal (Cilostazol) Tablets. Study 21‐86‐103. www.accessdata.fda.gov/drugsatfda_docs/nda/99/20863.cfm (accessed May 2014).
Otsuka Study 21‐87‐101 {unpublished data only}
-
- Otsuka America. Pletal (Cilostazol) Tablets. Study 21‐87‐101. www.accessdata.fda.gov/drugsatfda_docs/nda/99/20863.cfm (accessed May 2014).
Otsuka Study 21‐94‐301 {unpublished data only}
-
- Otsuka America. Pletal (Cilostazol) Tablets. Study 21‐94‐301. www.accessdata.fda.gov/drugsatfda_docs/nda/99/20863.cfm (accessed May 2014).
Otsuka Study 21‐95‐201 {unpublished data only}
-
- Otsuka 21‐95‐201. A randomised double‐blind study of the safety and efficacy of two different doses of cilostazol versus placebo in patients with intermittent claudication secondary to peripheral vascular disease. www.accessdata.fda.gov/drugsatfda_docs/nda/99/20863.cfm (accessed May 2014).
Otsuka Study 21‐98‐213 {unpublished data only}
-
- Anon. Pletal (cilostazol). Study 21‐98‐213 PACE. Otsuka Pharmaceuticals Submission to NICE: Cilostazol for the symptomatic treatment of intermittent claudication secondary to peripheral arterial disease 2010.
-
- European Medicines Agency. Assessment report for Cilostazol containing medicinal products. European Medicines Agency 2013.
Strandness 2002 {published data only}
-
- Otsuka 21‐94‐201. A randomised double blind study of the effect of cilostazol versus placebo on walking distances in patients with intermittent claudication secondary to peripheral vascular disease. www.accessdata.fda.gov/drugsatfda_docs/nda/99/20863.cfm (accessed May 2014).
-
- Strandness DE Jr, Dalman RL, Panian S, Rendell MS, Comp PC, Zhang P, et al. Effect of cilostazol in patients with intermittent claudication: a randomized, double‐blind, placebo‐controlled study. Vascular and Endovascular Surgery 2002;36(2):83‐91. - PubMed
References to studies excluded from this review
ACCELA {published and unpublished data}
-
- NCT00443287. Efficacy and safety of HMR1766 in patients with Fontaine Stage ii peripheral arterial disease. www.clinicaltrials.gov/ct2/show/NCT00443287 (accessed May 2014).
CASTLE {published data only}
-
- Hiatt WR, Money SR, Brass EP. Long‐term safety of cilostazol in patients with peripheral artery disease: The CASTLE study (Cilostazol: A study in long‐term effects). Journal of Vascular Surgery 2008;47(2):330‐6. - PubMed
-
- Otsuka 21‐98‐214‐01. CASTLE. A randomized, double‐blind, placebo‐controlled, multicenter, parallel‐arm, study to assess the long‐term effects of Pletal® (Cilostazol) versus placebo administered orally to patients with intermittent claudication secondary to peripheral arterial disease. www.accessdata.fda.gov/drugsatfda_docs/nda/99/20863.cfm (accessed May 2014).
-
- Stone WM, Demaerschalk BM, Fowl RJ, Money SR. Type 3 phosphodiesterase inhibitors may be protective against cerebrovascular events in patients with claudication. Journal of Stroke and Cerebrovascular Diseases 2008;17(3):129‐33. - PubMed
-
- Stone WM, Fowl RJ, Money SR. Type 3 phosphodiesterase inhibitors may be protective against cerebrovascular events in patients with claudication. Proceedings of the Vascular Annual Meeting; 2007 Jun 6‐10; Baltimore. 2007. - PubMed
Hsieh 2009 {published data only}
-
- Hsieh CJ, Wang PW. Effect of cilostazol treatment on adiponectin and soluble CD40 ligand levels in diabetic patients with peripheral arterial occlusion disease. Circulation Journal 2009;73(5):948‐54. - PubMed
Kim 2013 {published data only}
-
- Kim NH, Kim HY, An H, Seo JA, Kim NH, Choi KM, et al. Effect of cilostazol on arterial stiffness and vascular adhesion molecules in type 2 diabetic patients with metabolic syndrome: A randomised, double‐blind, placebo‐controlled, crossover trial. Diabetology and Metabolic Syndrome 2013;5(1):41. - PMC - PubMed
MASCOT {published and unpublished data}
-
- NCT00300339. MASCOT: Mixed antagonist of serotonin for claudication optimal therapy. www.clinicaltrials.gov/ct2/show/NCT00300339?term=MASCOT&rank=2 (accessed May 2014).
Otsuka Study PUIC‐1 {published data only}
-
- Otsuka America. Pletal (Cilostazol) Tablets. Study PUIC‐1. www.accessdata.fda.gov/drugsatfda_docs/nda/99/20863.cfm (accessed May 2014).
Otsuka Study PUIC‐2 {published data only}
-
- Otsuka America. Pletal (Cilostazol) Tablets. Study PUIC‐2. www.accessdata.fda.gov/drugsatfda_docs/nda/99/20863.cfm (accessed May 2014).
Samra 2003 {published data only}
-
- Samra SS, Bajaj P, Vijayaraghavan KS, Potdar NP, Vyas D, Devani RG, et al. Efficacy and safety of cilostazol, a novel phosphodiesterase inhibitor in patients with intermittent claudication. Journal of the Indian Medical Association 2003;101(9):561‐4. - PubMed
SPAD {published data only}
-
- NCT01188824. The safety and efficacy of cilostazol in ischemic stroke patients with peripheral arterial disease (SPAD study). clinicaltrials.gov/ct2/show/NCT01188824?term=SPAD&rank=3 (accessed May 2014).
Additional references
ATT 2002
Barnett 2004
-
- Barnett AH, Bradbury AW, Brittenden J, Crichton B, Donnelly R, Homer‐Vanniasinkam S, et al. The role of cilostazol in the treatment of intermittent claudication. Current Medical Research and Opinion 2004;20(10):1661‐70. - PubMed
Chapman 2003
-
- Chapman TM, Goa KL. Cilostazol: a review of its use in intermittent claudication. American Journal of Cardiovascular Drugs 2003;3(2):117‐38. - PubMed
Dawson 2001
-
- Dawson DL. Comparative effects of cilostazol and other therapies for intermittent claudication. American Journal of Cardiology 2001;87(12A):19D‐27D. - PubMed
Dormandy 1999
-
- Dormandy J, Heeck L, Vig S. The natural history of claudication: risk to life and limb. Seminars in Vascular Surgery 1999;12(2):123‐37. - PubMed
Fowkes 1998
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Iida 2008
-
- Iida O, Nanto S, Uematsu M, Morozumi T, Kitakaze M, Nagata S. Cilostazol reduces restenosis after endovascular therapy in patients with femoropopliteal lesions. Journal of Vascular Surgery 2008;48(1):144‐9. - PubMed
Khan 2005
-
- Khan S, Cleanthis M, Smout J, Flather M, Stansby G. Life‐style modification in peripheral arterial disease. European Journal of Vascular and Endovascular Surgery 2005;29(1):2‐9. - PubMed
Lee 2013
-
- Lee S‐W, Ahn J‐M, Han S, Park G‐M, Cho Y‐R, Lee W‐S, et al. Differential impact of cilostazol on restenosis according to implanted stent type (from a pooled analysis of three DECLARE randomized trials). American Journal of Cardiology 2013;112(9):1328‐34. - PubMed
Leng 1996
-
- Leng GC, Lee AJ, Fowkes FG, Whiteman M, Dunbar J, Housley E, et al. Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral artery disease in the general population. International Journal of Epidemiology 1996;25(6):1172‐81. - PubMed
NICE 2011
-
- National Institute for Health and Care Excellence (NICE). Cilostazol, naftidrofuryl oxalate, pentoxifylline and inositol nicotinate for the treatment of intermittent claudication in people with peripheral arterial disease. www.nice.org.uk/guidance/TA223/chapter/2‐Clinical‐need‐and‐practice (accessed 11 July 2014). - PubMed
NICE 2012
-
- National Institute for Health and Care Excellence (NICE). Lower limb peripheral arterial disease: diagnosis and management. www.nice.org.uk/Guidance/CG147 (accessed 11 July 2014).
PAD 2003
-
- Peripheral Arterial Diseases Antiplatelet Consensus Group. Antiplatelet therapy in peripheral arterial disease. Consensus statement. European Journal of Vascular and Endovascular Surgery 2003;26(1):1‐16. - PubMed
Pratt 2001
-
- Pratt CM. Analysis of the cilostazol safety database. American Journal of Cardiology 2001;87(12A):28D‐33D. - PubMed
Regensteiner 2002
-
- Regensteiner JG, Ware JE Jr, McCarthy WJ, Zhang P, Forbes WP, Heckman J, et al. Effect of cilostazol on treadmill walking, community‐based walking ability, and health‐related quality of life in patients with intermittent claudication due to peripheral arterial disease: meta‐analysis of six randomized controlled trials. Journal of the American Geriatrics Society 2002;50(12):1939‐46. - PubMed
Rizzo 2011
-
- Rizzo M, Corrado E, Patti AM, Rini GB, Mikhailidis DP. Cilostazol and atherogenic dyslipidemia: a clinically relevant effect?. Expert Opinion on Pharmacotherapy 2011;12(4):647‐55. - PubMed
Robless 2001
-
- Robless P, Mikhailidis D, Stansby G. Systematic review of antiplatelet therapy for prevention of myocardial infarction, stroke or vascular death in patients with peripheral vascular disease. British Journal of Surgery 2001;88(6):787‐800. - PubMed
Sallustio 2010
-
- Sallustio F, Rotondo F, Legge S, Stanzione P. Cilostazol in the management of atherosclerosis. Current Vascular Pharmacology 2010;8(3):363‐72. - PubMed
Squires 2010
-
- Squires H, Simpson E, Meng Y, Harnan S, Stevens J, Wong R, Technology Assessment Report commissioned by the NIHR HTA Programme on behalf of the National Institute for Health and Clinical Excellence. Cilostazol, naftidrofuryl oxalate, pentoxifylline and inositol nicotinate for intermittent claudication in people with peripheral arterial disease. www.guidance.nice.org.uk/nicemedia/live/12265/51580/51580.pdf (accessed May 2014). - PubMed
Squires 2011
-
- Squires H, Simpson E, Meng Y, Harnan S, Stevens J, Wong R, et al. A systematic review and economic evaluation of cilostazol, naftidrofuryl oxalate, pentoxifylline and inositol nicotinate for the treatment of intermittent claudication in people with peripheral arterial disease. Health Technology Assessment 2011;15(40):1‐210. [DOI: 10.3310/hta15400] - DOI - PMC - PubMed
Stevens 2012
-
- Stevens JW, Simpson E, Harnan S, Squires H, Meng Y, Thomas S, et al. Systematic review of the efficacy of cilostazol, naftidrofuryl oxalate and pentoxifylline for the treatment of intermittent claudication. British Journal of Surgery 2012;99(12):1630‐8. - PubMed
Thompson 2002
-
- Thompson PD, Zimet R, Forbes WP, Zhang P. Meta‐analysis of results from eight randomized, placebo‐controlled trials on the effect of cilostazol on patients with intermittent claudication. American Journal of Cardiology 2002;90(12):1314‐9. - PubMed
Ueno 2011
-
- Ueno H, Koyama H, Mima Y, Fukumoto S, Tanaka S, Shoji T, et al. Comparison of the effect of cilostazol with aspirin on circulating endothelial progenitor cells and small‐dense LDL cholesterol in diabetic patients with cerebral ischemia: a randomized controlled pilot trial. Journal of Atherosclerosis and Thrombosis 2011;18(10):883‐90. - PubMed
References to other published versions of this review
Robless 2007
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

