K-RasV14I recapitulates Noonan syndrome in mice
- PMID: 25359213
- PMCID: PMC4246321
- DOI: 10.1073/pnas.1418126111
K-RasV14I recapitulates Noonan syndrome in mice
Abstract
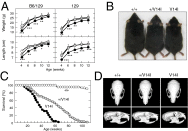
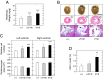
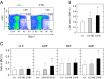
Noonan syndrome (NS) is an autosomal dominant genetic disorder characterized by short stature, craniofacial dysmorphism, and congenital heart defects. NS also is associated with a risk for developing myeloproliferative disorders (MPD), including juvenile myelomonocytic leukemia (JMML). Mutations responsible for NS occur in at least 11 different loci including KRAS. Here we describe a mouse model for NS induced by K-Ras(V14I), a recurrent KRAS mutation in NS patients. K-Ras(V14I)-mutant mice displayed multiple NS-associated developmental defects such as growth delay, craniofacial dysmorphia, cardiac defects, and hematologic abnormalities including a severe form of MPD that resembles human JMML. Homozygous animals had perinatal lethality whose penetrance varied with genetic background. Exposure of pregnant mothers to a MEK inhibitor rescued perinatal lethality and prevented craniofacial dysmorphia and cardiac defects. However, Mek inhibition was not sufficient to correct these defects when mice were treated after weaning. Interestingly, Mek inhibition did not correct the neoplastic MPD characteristic of these mutant mice, regardless of the timing at which the mice were treated, thus suggesting that MPD is driven by additional signaling pathways. These genetically engineered K-Ras(V14I)-mutant mice offer an experimental tool for studying the molecular mechanisms underlying the clinical manifestations of NS. Perhaps more importantly, they should be useful as a preclinical model to test new therapies aimed at preventing or ameliorating those deficits associated with this syndrome.
Keywords: MEK inhibitors; RASopathies; developmental disorders; heart defects; myeloproliferative disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Noonan JA, Ehmke DA. Associated noncardiac malformations in children with congenital heart disease. Midwest Soc Pediatr Res. 1963;63:468–470.
-
- Schubbert S, Shannon K, Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer. 2007;7(4):295–308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

