Homology-dependent repair is involved in 45S rDNA loss in plant CAF-1 mutants
- PMID: 25359579
- PMCID: PMC4309414
- DOI: 10.1111/tpj.12718
Homology-dependent repair is involved in 45S rDNA loss in plant CAF-1 mutants
Abstract
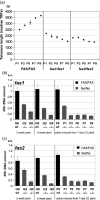
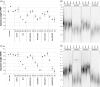
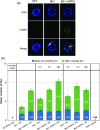
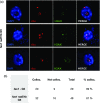
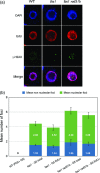
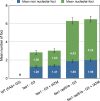
Arabidopsis thaliana mutants in FAS1 and FAS2 subunits of chromatin assembly factor 1 (CAF1) show progressive loss of 45S rDNA copies and telomeres. We hypothesized that homology-dependent DNA damage repair (HDR) may contribute to the loss of these repeats in fas mutants. To test this, we generated double mutants by crossing fas mutants with knock-out mutants in RAD51B, one of the Rad51 paralogs of A. thaliana. Our results show that the absence of RAD51B decreases the rate of rDNA loss, confirming the implication of RAD51B-dependent recombination in rDNA loss in the CAF1 mutants. Interestingly, this effect is not observed for telomeric repeat loss, which thus differs from that acting in rDNA loss. Involvement of DNA damage repair in rDNA dynamics in fas mutants is further supported by accumulation of double-stranded breaks (measured as γ-H2AX foci) in 45S rDNA. Occurrence of the foci is not specific for S-phase, and is ATM-independent. While the foci in fas mutants occur both in the transcribed (intranucleolar) and non-transcribed (nucleoplasmic) fraction of rDNA, double fas rad51b mutants show a specific increase in the number of the intranucleolar foci. These results suggest that the repair of double-stranded breaks present in the transcribed rDNA region is RAD51B dependent and that this contributes to rDNA repeat loss in fas mutants, presumably via the single-stranded annealing recombination pathway. Our results also highlight the importance of proper chromatin assembly in the maintenance of genome stability.
Keywords: 45S rDNA; Arabidopsis thaliana; DNA repair; FAS1; FAS2; RAD51B; chromatin assembly factor 1; genome instability.
© 2014 The Authors The Plant Journal published by Society for Experimental Biology and John Wiley & Sons Ltd.
Figures


References
-
- Alonso JM, Stepanova AN, Leisse TJ, et al. Genome-wide Insertional mutagenesis of Arabidopsis thaliana. Science. 2003;301:653–657. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

