Adipocyte amino acid sensing controls adult germline stem cell number via the amino acid response pathway and independently of Target of Rapamycin signaling in Drosophila
- PMID: 25359724
- PMCID: PMC4302921
- DOI: 10.1242/dev.116467
Adipocyte amino acid sensing controls adult germline stem cell number via the amino acid response pathway and independently of Target of Rapamycin signaling in Drosophila
Abstract
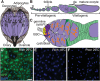
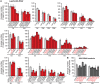
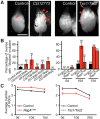
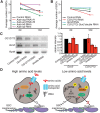
How adipocytes contribute to the physiological control of stem cells is a critical question towards understanding the link between obesity and multiple diseases, including cancers. Previous studies have revealed that adult stem cells are influenced by whole-body physiology through multiple diet-dependent factors. For example, nutrient-dependent pathways acting within the Drosophila ovary control the number and proliferation of germline stem cells (GSCs). The potential role of nutrient sensing by adipocytes in modulating stem cells in other organs, however, remains largely unexplored. Here, we report that amino acid sensing by adult adipocytes specifically modulates the maintenance of GSCs through a Target of Rapamycin-independent mechanism. Instead, reduced amino acid levels and the consequent increase in uncoupled tRNAs trigger activation of the GCN2-dependent amino acid response pathway within adipocytes, causing increased rates of GSC loss. These studies reveal a new step in adipocyte-stem cell crosstalk.
Keywords: Adipocytes; Amino acid transporters; Diet; Drosophila; Germline stem cells; Oogenesis.
© 2014. Published by The Company of Biologists Ltd.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

