ACCENT-based web calculators to predict recurrence and overall survival in stage III colon cancer
- PMID: 25359867
- PMCID: PMC4334801
- DOI: 10.1093/jnci/dju333
ACCENT-based web calculators to predict recurrence and overall survival in stage III colon cancer
Abstract
Background: Current prognostic tools in colon cancer use relatively few patient characteristics. We constructed and validated clinical calculators for overall survival (OS) and time to recurrence (TTR) for stage III colon cancer and compared their performance against an existing tool (Numeracy) and American Joint Committee on Cancer (AJCC) version 7 staging.
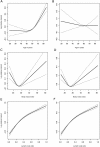
Methods: Data from 15936 stage III patients accrued to phase III clinical trials since 1989 were used to construct Cox models for TTR and OS. Variables included age, sex, race, body mass index, performance status, tumor grade, tumor stage, ratio of positive lymph nodes to nodes examined, number and location of primary tumors, and adjuvant treatment (fluoropyrimidine single agent or in combination). Missing data were imputed, and final models internally validated for optimism-corrected calibration and discrimination and compared with AJCC. External validation and comparisons against Numeracy were performed using stage III patients from NSABP trial C-08. All statistical tests were two-sided.
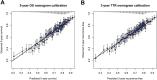
Results: All variables were statistically and clinically significant for OS prediction, while age and race did not predict TTR. No meaningful interactions existed. Models for OS and TTR were well calibrated and associated with C-indices of 0.66 and 0.65, respectively, compared with C-indices of 0.58 and 0.59 for AJCC. These tools, available online, better predicted patient outcomes than Numeracy, both overall and within patient subgroups, in external validation.
Conclusions: The proposed ACCENT calculators are internally and externally valid, better discriminate patient risk than AJCC version 7 staging, and better predict patient outcomes than Numeracy. These tools have replaced Numeracy for online clinical use and will aid prognostication and patient/physician communication.
© The Author 2014. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- CDC – Colorectal Cancer Statistics. Centers for Disease Control and Prevention. Atlanta, GA: Available at: http://www.cdc.gov/cancer/colorectal/statistics Accessed March 22, 2013.
-
- André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350(23):2343–2351. - PubMed
-
- André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27(19):3109–3116. - PubMed
-
- Schmoll H-J, Cartwright T, Tabernero J, et al. Phase III trial of capecitabine plus oxaliplatin as adjuvant therapy for stage III colon cancer: a planned safety analysis in 1,864 patients. J Clin Oncol. 2006;25(1):102–109. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

