Fluid Mechanics, Arterial Disease, and Gene Expression
- PMID: 25360054
- PMCID: PMC4211638
- DOI: 10.1146/annurev-fluid-010313-141309
Fluid Mechanics, Arterial Disease, and Gene Expression
Abstract
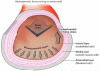
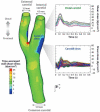
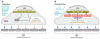
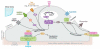
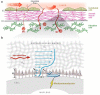
This review places modern research developments in vascular mechanobiology in the context of hemodynamic phenomena in the cardiovascular system and the discrete localization of vascular disease. The modern origins of this field are traced, beginning in the 1960s when associations between flow characteristics, particularly blood flow-induced wall shear stress, and the localization of atherosclerotic plaques were uncovered, and continuing to fluid shear stress effects on the vascular lining endothelial) cells (ECs), including their effects on EC morphology, biochemical production, and gene expression. The earliest single-gene studies and genome-wide analyses are considered. The final section moves from the ECs lining the vessel wall to the smooth muscle cells and fibroblasts within the wall that are fluid me chanically activated by interstitial flow that imposes shear stresses on their surfaces comparable with those of flowing blood on EC surfaces. Interstitial flow stimulates biochemical production and gene expression, much like blood flow on ECs.
Keywords: endothelial cells; glycocalyx; interstitial flow; mechanotransduction; shear stress; smooth muscle cells.
Figures

References
-
- Abumiya T, Sasaguri T, Taba Y, Miwa Y, Miyagi M. Shear stress induces expression of vascular endothelial growth factor receptor Flk-1/KDR through the CT-rich Sp1 binding site. Arterioscler. Thromb. Vasc. Biol. 2002;22:907–13. - PubMed
-
- Ainslie KM, Garanich JS, Dull RO, Tarbell JM. Vascular smooth muscle cell glycocalyx influences shear stress-mediated contractile response. J. Appl. Physiol. 2005;98:242–49. - PubMed
-
- Akimoto S, Mitsumata M, Sasaguri T, Yoshida Y. Laminar shear stress inhibits vascular endothelial cell proliferation by inducing cyclin-dependent kinase inhibitor p21Sdi1/Cip1/Waf1. Circ. Res. 2000;86:185–90. - PubMed
-
- Alshihabi SN, Chang YS, Frangos JA, Tarbell JM. Shear stress-induced release of PGE2 and PGI2 by vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 1996;224:808–14. - PubMed
-
- Apenberg S, Freyberg MA, Friedl P. Shear stress induces apoptosis in vascular smooth muscle cells via an autocrine Fas/FasL pathway. Biochem. Biophys. Res. Commun. 2003;310:355–59. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
