Enhancement of median nerve regeneration by mesenchymal stem cells engraftment in an absorbable conduit: improvement of peripheral nerve morphology with enlargement of somatosensory cortical representation
- PMID: 25360086
- PMCID: PMC4199278
- DOI: 10.3389/fnana.2014.00111
Enhancement of median nerve regeneration by mesenchymal stem cells engraftment in an absorbable conduit: improvement of peripheral nerve morphology with enlargement of somatosensory cortical representation
Abstract
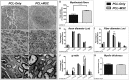
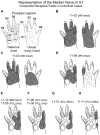
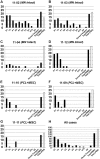
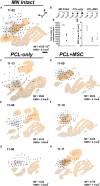
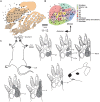
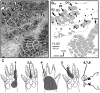
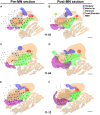
We studied the morphology and the cortical representation of the median nerve (MN), 10 weeks after a transection immediately followed by treatment with tubulization using a polycaprolactone (PCL) conduit with or without bone marrow-derived mesenchymal stem cell (MSC) transplant. In order to characterize the cutaneous representation of MN inputs in primary somatosensory cortex (S1), electrophysiological cortical mapping of the somatosensory representation of the forepaw and adjacent body parts was performed after acute lesion of all brachial plexus nerves, except for the MN. This was performed in ten adult male Wistar rats randomly assigned in three groups: MN Intact (n = 4), PCL-Only (n = 3), and PCL+MSC (n = 3). Ten weeks before mapping procedures in animals from PCL-Only and PCL+MSC groups, animal were subjected to MN transection with removal of a 4-mm-long segment, immediately followed by suturing a PCL conduit to the nerve stumps with (PCL+MSC group) or without (PCL-Only group) injection of MSC into the conduit. After mapping the representation of the MN in S1, animals had a segment of the regenerated nerve processed for light and transmission electron microscopy. For histomorphometric analysis of the nerve segment, sample size was increased to five animals per experimental group. The PCL+MSC group presented a higher number of myelinated fibers and a larger cortical representation of MN inputs in S1 (3,383 ± 390 fibers; 2.3 mm(2), respectively) than the PCL-Only group (2,226 ± 575 fibers; 1.6 mm(2)). In conclusion, MSC-based therapy associated with PCL conduits can improve MN regeneration. This treatment seems to rescue the nerve representation in S1, thus minimizing the stabilization of new representations of adjacent body parts in regions previously responsive to the MN.
Keywords: cortical plasticity; median nerve lesion; nerve regeneration; polycaprolactone conduits; rat; stem-cell therapy.
Figures
References
-
- Anomal R. F., Rocha-Rego V., Franca J. G. (2011). Topographic organization and corticocortical connections of the forepaw representation in areas S1 and SC of the opossum: evidence for a possible role of area SC in multimodal processing. Front. Neuroanat. 5:56. 10.3389/fnana.2011.00056 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

