Differential loss of thalamostriatal and corticostriatal input to striatal projection neuron types prior to overt motor symptoms in the Q140 knock-in mouse model of Huntington's disease
- PMID: 25360089
- PMCID: PMC4197654
- DOI: 10.3389/fnsys.2014.00198
Differential loss of thalamostriatal and corticostriatal input to striatal projection neuron types prior to overt motor symptoms in the Q140 knock-in mouse model of Huntington's disease
Abstract
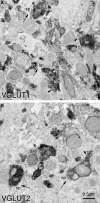
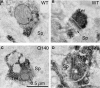
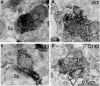
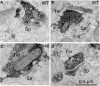
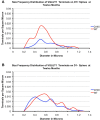
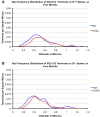
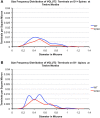
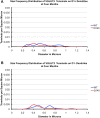
Motor slowing and forebrain white matter loss have been reported in premanifest Huntington's disease (HD) prior to substantial striatal neuron loss. These findings raise the possibility that early motor defects in HD may be related to loss of excitatory input to striatum. In a prior study, we showed that in the heterozygous Q140 knock-in mouse model of HD that loss of thalamostriatal axospinous terminals is evident by 4 months, and loss of corticostriatal axospinous terminals is evident at 12 months, before striatal projection neuron pathology. In the present study, we specifically characterized the loss of thalamostriatal and corticostriatal terminals on direct (dSPN) and indirect (iSPN) pathway striatal projection neurons, using immunolabeling to identify thalamostriatal (VGLUT2+) and corticostriatal (VGLUT1+) axospinous terminals, and D1 receptor immunolabeling to distinguish dSPN (D1+) and iSPN (D1-) synaptic targets. We found that the loss of corticostriatal terminals at 12 months of age was preferential for D1+ spines, and especially involved smaller terminals, presumptively of the intratelencephalically projecting (IT) type. By contrast, indirect pathway D1- spines showed little loss of axospinous terminals at the same age. Thalamostriatal terminal loss was comparable for D1+ and D1- spines at both 4 and 12 months. Regression analysis showed that the loss of VGLUT1+ terminals on D1+ spines was correlated with a slight decline in open field motor parameters at 12 months. Our overall results raise the possibility that differential thalamic and cortical input loss to SPNs is an early event in human HD, with cortical loss to dSPNs in particular contributing to premanifest motor slowing.
Keywords: Huntington's disease; corticostriatal; pathology; premanifest; thalamostriatal.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

