Analogous reserve distribution and tissue characteristics in quinoa and grass seeds suggest convergent evolution
- PMID: 25360139
- PMCID: PMC4199267
- DOI: 10.3389/fpls.2014.00546
Analogous reserve distribution and tissue characteristics in quinoa and grass seeds suggest convergent evolution
Abstract
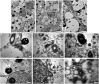
Quinoa seeds are highly nutritious due to the quality of their proteins and lipids and the wide range of minerals and vitamins they store. Three compartments can be distinguished within the mature seed: embryo, endosperm, and perisperm. The distribution of main storage reserves is clearly different in those areas: the embryo and endosperm store proteins, lipids, and minerals, and the perisperm stores starch. Tissues equivalent (but not homologous) to those found in grasses can be identified in quinoa, suggesting the effectiveness of this seed reserve distribution strategy; as in cells of grass starchy endosperm, the cells of the quinoa perisperm endoreduplicate, increase in size, synthesize starch, and die during development. In addition, both systems present an extra-embryonic tissue that stores proteins, lipids and minerals: in gramineae, the aleurone layer(s) of the endosperm; in quinoa, the micropylar endosperm; in both cases, the tissues are living. Moreover, the quinoa micropylar endosperm and the coleorhiza in grasses play similar roles, protecting the root in the quiescent seed and controlling dormancy during germination. This investigation is just the beginning of a broader and comparative study of the development of quinoa and grass seeds. Several questions arise from this study, such as: how are synthesis and activation of seed proteins and enzymes regulated during development and germination, what are the genes involved in these processes, and lastly, what is the genetic foundation justifying the analogy to grasses.
Keywords: coleorhiza; endosperm; grass seed; micropylar endosperm; perisperm; quinoa seed.
Figures







References
-
- Arana M. V., Burgin M. J., de Miguel L. C., Sánchez R. A. (2007). The very-low-fluence and high-irradiance responses of the phytochromes have antagonistic effects on germination, mannan-degrading activities, and DfGA3ox transcript levels in Datura ferox seeds. J. Exp. Bot. 58 3997–4004. 10.1093/jxb/erm256 - DOI - PubMed
-
- Balzotti M. R. B., Thornton J. N., Maughan P. J., McClellan D. A., Stevens M. R., Jellen E. N., et al. (2008). Expression and evolutionary relationship of the Chenopodium quinoa 11S seed storage protein gene. Int. J. Plant Sci. 169 281–291. 10.1086/523874 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

