The multi-protein family of sulfotransferases in plants: composition, occurrence, substrate specificity, and functions
- PMID: 25360143
- PMCID: PMC4199319
- DOI: 10.3389/fpls.2014.00556
The multi-protein family of sulfotransferases in plants: composition, occurrence, substrate specificity, and functions
Abstract
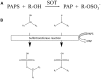
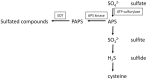
All members of the sulfotransferase (SOT, EC 2.8.2.-) protein family transfer a sulfuryl group from the donor 3'-phosphoadenosine 5'-phosphosulfate (PAPS) to an appropriate hydroxyl group of several classes of substrates. The primary structure of these enzymes is characterized by a histidine residue in the active site, defined PAPS binding sites and a longer SOT domain. Proteins with this SOT domain occur in all organisms from all three domains, usually as a multi-protein family. Arabidopsis thaliana SOTs, the best characterized SOT multi-protein family, contains 21 members. The substrates for several plant enzymes have already been identified, such as glucosinolates, brassinosteroids, jasmonates, flavonoids, and salicylic acid. Much information has been gathered on desulfo-glucosinolate (dsGl) SOTs in A. thaliana. The three cytosolic dsGl SOTs show slightly different expression patterns. The recombinant proteins reveal differences in their affinity to indolic and aliphatic dsGls. Also the respective recombinant dsGl SOTs from different A. thaliana ecotypes differ in their kinetic properties. However, determinants of substrate specificity and the exact reaction mechanism still need to be clarified. Probably, the three-dimensional structures of more plant proteins need to be solved to analyze the mode of action and the responsible amino acids for substrate binding. In addition to A. thaliana, more plant species from several families need to be investigated to fully elucidate the diversity of sulfated molecules and the way of biosynthesis catalyzed by SOT enzymes.
Keywords: Arabidopsis thaliana; glucosinolate; histidine residue; phosphoadenosine 5′-phosphosulfate; sulfotransferase.
Figures

References
-
- Alexandratos S. D. (1999). Synthesis and purification of zosteric acid. US Patent 5990336.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

