Probing the immune and healing response of murine intestinal mucosa by time-lapse 2-photon microscopy of laser-induced lesions with real-time dosimetry
- PMID: 25360369
- PMCID: PMC4206321
- DOI: 10.1364/BOE.5.003521
Probing the immune and healing response of murine intestinal mucosa by time-lapse 2-photon microscopy of laser-induced lesions with real-time dosimetry
Abstract
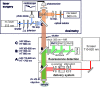
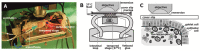
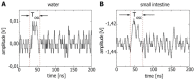
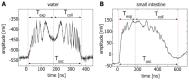
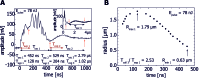
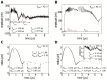
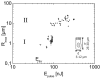
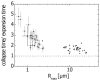
Gut mucosa is an important interface between body and environment. Immune response and healing processes of murine small intestinal mucosa were investigated by intravital time-lapse two-photon excited autofluorescence microscopy of the response to localized laser-induced damage. Epithelial lesions were created by 355-nm, 500-ps pulses from a microchip laser that produced minute cavitation bubbles. Size and dynamics of these bubbles were monitored using a novel interferometric backscattering technique with 80 nm resolution. Small bubbles (< 2.5 µm maximum radius) merely resulted in autofluorescence loss of the target cell. Larger bubbles (7-25 µm) affected several cells and provoked immigration of immune cells (polymorphonuclear leucocytes). Damaged cells were expelled into the lumen, and the epithelium healed within 2 hours by stretching and migration of adjacent epithelial cells.
Keywords: (170.1020) Ablation of tissue; (170.2520) Fluorescence microscopy; (170.2680) Gastrointestinal; (170.3880) Medical and biological imaging; (170.4520) Optical confinement and manipulation; (180.4315) Nonlinear microscopy; (350.3390) Laser materials processing; (350.4855) Optical tweezers or optical manipulation.
Figures







Similar articles
-
Intravital autofluorescence 2-photon microscopy of murine intestinal mucosa with ultra-broadband femtosecond laser pulse excitation: image quality, photodamage, and inflammation.J Biomed Opt. 2015 Nov;20(11):116001. doi: 10.1117/1.JBO.20.11.116001. J Biomed Opt. 2015. PMID: 26524678
-
Time-resolved observations of shock waves and cavitation bubbles generated by femtosecond laser pulses in corneal tissue and water.Lasers Surg Med. 1996;19(1):23-31. doi: 10.1002/(SICI)1096-9101(1996)19:1<23::AID-LSM4>3.0.CO;2-S. Lasers Surg Med. 1996. PMID: 8836993
-
Probing mesoscopic process of laser ablation in liquid by integrated method of optical beam deflection and time-resolved shadowgraphy.J Colloid Interface Sci. 2017 Mar 1;489:38-46. doi: 10.1016/j.jcis.2016.09.032. Epub 2016 Sep 15. J Colloid Interface Sci. 2017. PMID: 27823761
-
Intravital multidimensional real-time imaging of the conjunctival immune system.Dev Ophthalmol. 2010;45:40-48. doi: 10.1159/000315018. Epub 2010 May 18. Dev Ophthalmol. 2010. PMID: 20502025 Review.
-
Insights From Dynamic Neuro-Immune Imaging on Murine Immune Responses to CNS Damage.Front Neurosci. 2019 Jul 17;13:737. doi: 10.3389/fnins.2019.00737. eCollection 2019. Front Neurosci. 2019. PMID: 31379488 Free PMC article. Review.
Cited by
-
Longitudinal imaging and femtosecond laser manipulation of the liver: How to generate and trace single-cell-resolved micro-damage in vivo.PLoS One. 2020 Oct 15;15(10):e0240405. doi: 10.1371/journal.pone.0240405. eCollection 2020. PLoS One. 2020. PMID: 33057345 Free PMC article.
-
Femtosecond laser bone ablation with a high repetition rate fiber laser source.Biomed Opt Express. 2014 Dec 5;6(1):32-42. doi: 10.1364/BOE.6.000032. eCollection 2015 Jan 1. Biomed Opt Express. 2014. PMID: 25657872 Free PMC article.
-
Spectrum- and time-resolved endogenous multiphoton signals reveal quantitative differentiation of premalignant and malignant gastric mucosa.Biomed Opt Express. 2018 Jan 8;9(2):453-471. doi: 10.1364/BOE.9.000453. eCollection 2018 Feb 1. Biomed Opt Express. 2018. PMID: 29552386 Free PMC article.
-
Illuminating Immunity: A Systematic Review of Immune Cell Autofluorescence.J Biophotonics. 2025 Jun;18(6):e202400576. doi: 10.1002/jbio.202400576. Epub 2025 Mar 20. J Biophotonics. 2025. PMID: 40112892 Free PMC article.
References
-
- Moore R., Carlson S., Madara J. L., “Rapid barrier restitution in an in vitro model of intestinal epithelial injury,” Lab. Invest. 60(2), 237–244 (1989). - PubMed
-
- McCormack S. A., Viar M. J., Johnson L. R., “Migration of IEC-6 cells: a model for mucosal healing,” Am. J. Physiol. 263(3), G426–G435 (1992). - PubMed
-
- Derikx J. P. M., Matthijsen R. A., De Bruïne A. P., Van Bijnen A. A., Heineman E., Van Dam R. M., Dejong C. H. C., Buurman W. A., “Rapid reversal of human intestinal ischemia-reperfusion induced damage by shedding of injured enterocytes and reepithelialisation,” PLoS ONE 3(10), e3428 (2008).10.1371/journal.pone.0003428 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
