Pharmacological induction of cell surface GRP78 contributes to apoptosis in triple negative breast cancer cells
- PMID: 25360516
- PMCID: PMC4294336
- DOI: 10.18632/oncotarget.2576
Pharmacological induction of cell surface GRP78 contributes to apoptosis in triple negative breast cancer cells
Abstract
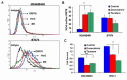
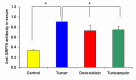
Breast cancer tumor with triple-negative receptors (estrogen, progesterone and Her 2, receptors) is the most aggressive and deadly subtype, with high rates of disease recurrence and poor survival. Here, we show that induction in cell surface GRP78 by doxorubicin and tunicamycin was associated with CHOP/GADD153 upregulation and increase in apoptosis in triple negative breast cancer tumor cells. GRP78 is a major regulator of the stress induced unfolded protein response pathway and CHOP/GADD153 is a pro-apoptotic transcription factor associated exclusively with stress induced apoptosis. The blocking of cell surface GRP78 by anti-GRP78 antibody prevented apoptosis, suggesting that induction of cell surface GRP78 by doxorubicin and tunicamycin is required for apoptosis. A better understanding of stress induction of apoptotic signaling in triple negative breast cancer cells may help to define new therapeutic strategies.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Chemotherapy-Induced Cell-Surface GRP78 Expression as a Prognostic Marker for Invasiveness of Metastatic Triple-Negative Breast Cancer.Ann Biomed Eng. 2025 Apr;53(4):881-890. doi: 10.1007/s10439-024-03673-z. Epub 2025 Jan 5. Ann Biomed Eng. 2025. PMID: 39757331 Free PMC article.
-
Structure-activity relationship of piperine and its synthetic amide analogs for therapeutic potential to prevent experimentally induced ER stress in vitro.Cell Stress Chaperones. 2017 May;22(3):417-428. doi: 10.1007/s12192-017-0786-9. Epub 2017 Apr 10. Cell Stress Chaperones. 2017. PMID: 28397086 Free PMC article.
-
Targeting HSP70 and GRP78 in canine osteosarcoma cells in combination with doxorubicin chemotherapy.Cell Stress Chaperones. 2016 Nov;21(6):1065-1076. doi: 10.1007/s12192-016-0730-4. Epub 2016 Sep 8. Cell Stress Chaperones. 2016. PMID: 27631331 Free PMC article.
-
The Glucose-Regulated Protein78 (GRP78) in the Unfolded Protein Response (UPR) Pathway: A Potential Therapeutic Target for Breast Cancer.Anticancer Agents Med Chem. 2023;23(5):505-524. doi: 10.2174/1871520622666220823094350. Anticancer Agents Med Chem. 2023. PMID: 36017846 Review.
-
Immunoglobulin-binding protein and Toll-like receptors in immune landscape of breast cancer.Life Sci. 2024 Dec 1;358:123196. doi: 10.1016/j.lfs.2024.123196. Epub 2024 Oct 30. Life Sci. 2024. PMID: 39481836 Review.
Cited by
-
The Clinicopathological Significance of BiP/GRP-78 in Breast Cancer: A Meta-Analysis of Public Datasets and Immunohistochemical Detection.Curr Oncol. 2022 Nov 23;29(12):9066-9087. doi: 10.3390/curroncol29120710. Curr Oncol. 2022. PMID: 36547124 Free PMC article.
-
GRP78 expression in peripheral blood mononuclear cells is a new predictive marker for the benefit of taxanes in breast cancer neoadjuvant treatment.BMC Cancer. 2020 Apr 19;20(1):333. doi: 10.1186/s12885-020-06835-z. BMC Cancer. 2020. PMID: 32306920 Free PMC article.
-
Cell surface GRP78: a potential mechanism of therapeutic resistant tumors.Cancer Cell Int. 2023 May 23;23(1):100. doi: 10.1186/s12935-023-02931-9. Cancer Cell Int. 2023. PMID: 37221596 Free PMC article. Review.
-
Cell surface GRP78: A potential marker of good prognosis and response to chemotherapy in breast cancer.Oncol Lett. 2015 Oct;10(4):2149-2155. doi: 10.3892/ol.2015.3579. Epub 2015 Aug 6. Oncol Lett. 2015. PMID: 26622810 Free PMC article.
-
Blocking heme oxygenase-1 by zinc protoporphyrin reduces tumor hypoxia-mediated VEGF release and inhibits tumor angiogenesis as a potential therapeutic agent against colorectal cancer.J Biomed Sci. 2016 Jan 28;23:18. doi: 10.1186/s12929-016-0219-6. J Biomed Sci. 2016. PMID: 26822586 Free PMC article.
References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- DeSantis C, Siegel R, Bandi P, Jemal A. Breast cancer statistics, 2011. CA Cancer J Clin. 2011;61:409–418. - PubMed
-
- Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lønning PE, Børresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. - PubMed
-
- Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

