LRRK2 transport is regulated by its novel interacting partner Rab32
- PMID: 25360523
- PMCID: PMC4216093
- DOI: 10.1371/journal.pone.0111632
LRRK2 transport is regulated by its novel interacting partner Rab32
Abstract
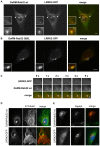
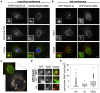
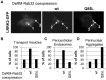
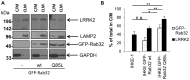
Leucine-rich repeat kinase 2 (LRRK2) is a multi-domain 280 kDa protein that is linked to Parkinson's disease (PD). Mutations especially in the GTPase and kinase domains of LRRK2 are the most common causes of heritable PD and are also found in sporadic forms of PD. Although the cellular function of LRRK2 is largely unknown there is increasing evidence that these mutations cause cell death due to autophagic dysfunction and mitochondrial damage. Here, we demonstrate a novel mechanism of LRRK2 binding and transport, which involves the small GTPases Rab32 and Rab38. Rab32 and its closest homologue Rab38 are known to organize the trans-Golgi network and transport of key enzymes in melanogenesis, whereas their function in non-melanogenic cells is still not well understood. Cellular processes such as autophagy, mitochondrial dynamics, phagocytosis or inflammatory processes in the brain have previously been linked to Rab32. Here, we demonstrate that Rab32 and Rab38, but no other GTPase tested, directly interact with LRRK2. GFP-Trap analyses confirmed the interaction of Rab32 with the endogenous LRRK2. In yeast two-hybrid experiments we identified a predicted coiled-coil motif containing region within the aminoterminus of LRRK2 as the possible interacting domain. Fluorescence microscopy demonstrated a co-localization of Rab32 and LRRK2 at recycling endosomes and transport vesicles, while overexpression of a constitutively active mutant of Rab32 led to an increased co-localization with Rab7/9 positive perinuclear late endosomes/MVBs. Subcellular fractionation experiments supported the novel role of Rab32 in LRRK2 late endosomal transport and sorting in the cell. Thus, Rab32 may regulate the physiological functions of LRRK2.
Conflict of interest statement
Figures


References
-
- Gloeckner CJ, Kinkl N, Schumacher A, Braun RJ, O'Neill E, et al. (2006) The Parkinson disease causing LRRK2 mutation I2020T is associated with increased kinase activity. Hum Mol Genet 15: 223–232. - PubMed
-
- Santpere G, Ferrer I (2009) LRRK2 and neurodegeneration. Acta Neuropathol 117: 227–246. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

