Multiparametric magnetic resonance imaging for predicting pathological response after the first cycle of neoadjuvant chemotherapy in breast cancer
- PMID: 25360603
- PMCID: PMC4471951
- DOI: 10.1097/RLI.0000000000000100
Multiparametric magnetic resonance imaging for predicting pathological response after the first cycle of neoadjuvant chemotherapy in breast cancer
Abstract
Objectives: The purpose of this study was to determine whether multiparametric magnetic resonance imaging (MRI) using dynamic contrast-enhanced MRI (DCE-MRI) and diffusion-weighted MRI (DWI), obtained before and after the first cycle of neoadjuvant chemotherapy (NAC), is superior to single-parameter measurements for predicting pathologic complete response (pCR) in patients with breast cancer.
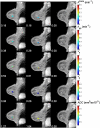
Materials and methods: Patients with stage II/III breast cancer were enrolled in an institutional review board-approved study in which 3-T DCE-MRI and DWI data were acquired before (n = 42) and after 1 cycle (n = 36) of NAC. Estimates of the volume transfer rate (K), extravascular extracellular volume fraction (ve), blood plasma volume fraction (vp), and the efflux rate constant (kep = K/ve) were generated from the DCE-MRI data using the Extended Tofts-Kety model. The apparent diffusion coefficient (ADC) was estimated from the DWI data. The derived parameter kep/ADC was compared with single-parameter measurements for its ability to predict pCR after the first cycle of NAC.
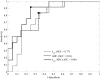
Results: The kep/ADC after the first cycle of NAC discriminated patients who went on to achieve a pCR (P < 0.001) and achieved a sensitivity, specificity, positive predictive value, and area under the receiver operator curve (AUC) of 0.92, 0.78, 0.69, and 0.88, respectively. These values were superior to the single parameters kep (AUC, 0.76) and ADC (AUC, 0.82). The AUCs between kep/ADC and kep were significantly different on the basis of the bootstrapped 95% confidence intervals (0.018-0.23), whereas the AUCs between kep/ADC and ADC trended toward significance (-0.11 to 0.24).
Conclusions: The multiparametric analysis of DCE-MRI and DWI was superior to the single-parameter measurements for predicting pCR after the first cycle of NAC.
Figures

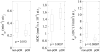

References
-
- Guo Y, Cai YQ, Cai ZL, Gao YG, An NY, Ma L, Mahankali S, Gao JH. Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging. J Magn Reson Imaging. 2002;16:172–178. - PubMed
-
- Cheung YC, Chen SC, Su MY, See LC, Hsueh S, Chang HK, Lin YC, Tsai CS. Monitoring the size and response of locally advanced breast cancers to neoadjuvant chemotherapy (weekly paclitaxel and epirubicin) with serial enhanced MRI. Breast Cancer Res Treat. 2003;78:51–58. - PubMed
-
- Chou CP, Wu MT, Chang HT, Lo YS, Pan HB, Degani H, Furman-Haran E. Monitoring breast cancer response to neoadjuvant systemic chemotherapy using parametric contrast-enhanced MRI: a pilot study. Acad Radiol. 2007;14:561–573. - PubMed
-
- Martincich L, Montemurro F, De Rosa G, Marra V, Ponzone R, Cirillo S, Gatti M, Biglia N, Sarotto I, Sismondi P, Regge D, Aglietta M. Monitoring response to primary chemotherapy in breast cancer using dynamic contrast-enhanced magnetic resonance imaging. Breast Cancer Res Treat. 2004;83:67–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

