Establishment of a rabbit Oct4 promoter-based EGFP reporter system
- PMID: 25360692
- PMCID: PMC4215976
- DOI: 10.1371/journal.pone.0109728
Establishment of a rabbit Oct4 promoter-based EGFP reporter system
Abstract
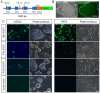
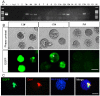
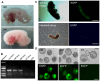
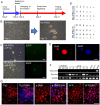
Rabbits are commonly used as laboratory animal models to investigate human diseases and phylogenetic development. However, pluripotent stem cells that contribute to germline transmission have yet to be established in rabbits. The transcription factor Oct4, also known as Pou5f1, is considered essential for the maintenance of the pluripotency of stem cells. Hence, pluripotent cells can be identified by monitoring Oct4 expression using a well-established Oct4 promoter-based reporter system. This study developed a rabbit Oct4 promoter-based enhanced green fluorescent protein (EGFP) reporter system by transfecting pROP2-EGFP into rabbit fetal fibroblasts (RFFs). The transgenic RFFs were used as donor cells for somatic cell nuclear transfer (SCNT). The EGFP expression was detected in the blastocysts and genital ridges of SCNT fetuses. Fibroblasts and neural stem cells (NSCs) were derived from the SCNT fetuses. EGFP was also reactivated in blastocysts after the second SCNT, and induced pluripotent stem cells (iPSCs) were obtained after reprogramming using Yamanaka's factors. The results above indicated that a rabbit reporter system used to monitor the differentiating status of cells was successfully developed.
Conflict of interest statement
Figures
References
-
- Liao J, Cui C, Chen S, Ren J, Chen J, et al. (2009) Generation of induced pluripotent stem cell lines from adult rat cells. Cell Stem Cell 4: 11–15. - PubMed
-
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, et al. (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861–872. - PubMed
-
- Takahashi K, Yamanaka S (2006) Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 126: 663–676. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

