Enterovirus-71 virus-like particles induce the activation and maturation of human monocyte-derived dendritic cells through TLR4 signaling
- PMID: 25360749
- PMCID: PMC4216083
- DOI: 10.1371/journal.pone.0111496
Enterovirus-71 virus-like particles induce the activation and maturation of human monocyte-derived dendritic cells through TLR4 signaling
Abstract
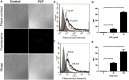
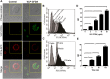
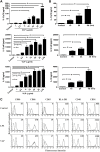
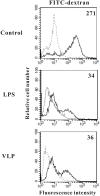
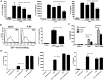
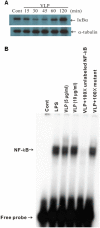
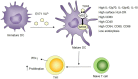
Enterovirus 71 (EV71) causes seasonal epidemics of hand-foot-and-mouth disease and has a high mortality rate among young children. We recently demonstrated potent induction of the humoral and cell-mediated immune response in monkeys immunized with EV71 virus-like particles (VLPs), with a morphology resembling that of infectious EV71 virions but not containing a viral genome, which could potentially be safe as a vaccine for EV71. To elucidate the mechanisms through which EV71 VLPs induce cell-mediated immunity, we studied the immunomodulatory effects of EV71 VLPs on human monocyte-derived dendritic cells (DCs), which bind to and incorporate EV71 VLPs. DC treatment with EV71 VLPs enhanced the expression of CD80, CD86, CD83, CD40, CD54, and HLA-DR on the cell surface; increased the production of interleukin (IL)-12 p40, IL-12 p70, and IL-10 by DCs; and suppressed the capacity of DCs for endocytosis. Treatment with EV71 VLPs also enhanced the ability of DCs to stimulate naïve T cells and induced secretion of interferon (IFN)-γ by T cells and Th1 cell responses. Neutralization with antibodies against Toll-like receptor (TLR) 4 suppressed the capacity of EV71 VLPs to induce the production of IL-12 p40, IL-12 p70, and IL-10 by DCs and inhibited EV71 VLPs binding to DCs. Our study findings clarified the important role for TLR4 signaling in DCs in response to EV71 VLPs and showed that EV71 VLPs induced inhibitor of kappaB alpha (IκBα) degradation and nuclear factor of kappaB (NF-κB) activation.
Conflict of interest statement
Figures

References
-
- Tagaya I, Takayama R, Hagiwara A (1981) A large-scale epidemic of hand, foot and mouth disease associated with enterovirus 71 infection in Japan in 1978. Jpn J Med Sci Biol 34: 191–196. - PubMed
-
- Wang SM, Lei HY, Yu CK, Wang JR, Su IJ, et al. (2008) Acute chemokine response in the blood and cerebrospinal fluid of children with enterovirus 71-associated brainstem encephalitis. J Infect Dis 198: 1002–1006. - PubMed
-
- Wu CN, Lin YC, Fann C, Liao NS, Shih SR, et al. (2001) Protection against lethal enterovirus 71 infection in newborn mice by passive immunization with subunit VP1 vaccines and inactivated virus. Vaccine 20: 895–904. - PubMed
-
- McMinn PC (2002) An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol Rev 26: 91–107. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

