Lichen secondary metabolites in Flavocetraria cucullata exhibit anti-cancer effects on human cancer cells through the induction of apoptosis and suppression of tumorigenic potentials
- PMID: 25360754
- PMCID: PMC4216107
- DOI: 10.1371/journal.pone.0111575
Lichen secondary metabolites in Flavocetraria cucullata exhibit anti-cancer effects on human cancer cells through the induction of apoptosis and suppression of tumorigenic potentials
Expression of concern in
-
Expression of Concern: Lichen Secondary Metabolites in Flavocetraria cucullata Exhibit Anti-Cancer Effects on Human Cancer Cells through the Induction of Apoptosis and Suppression of Tumorigenic Potentials.PLoS One. 2023 Feb 24;18(2):e0282452. doi: 10.1371/journal.pone.0282452. eCollection 2023. PLoS One. 2023. PMID: 36827410 Free PMC article. No abstract available.
Abstract
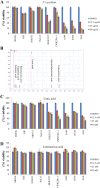
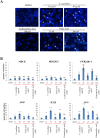
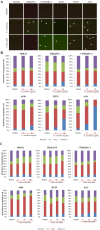
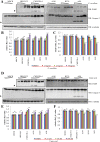
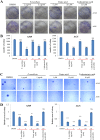
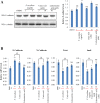
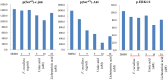
Lichens are symbiotic organisms which produce distinct secondary metabolic products. In the present study, we tested the cytotoxic activity of 17 lichen species against several human cancer cells and further investigated the molecular mechanisms underlying their anti-cancer activity. We found that among 17 lichens species, F. cucullata exhibited the most potent cytotoxicity in several human cancer cells. High performance liquid chromatography analysis revealed that the acetone extract of F. cucullata contains usnic acid, salazinic acid, Squamatic acid, Baeomycesic acid, d-protolichesterinic acid, and lichesterinic acid as subcomponents. MTT assay showed that cancer cell lines were more vulnerable to the cytotoxic effects of the extract than non-cancer cell lines. Furthermore, among the identified subcomponents, usnic acid treatment had a similar cytotoxic effect on cancer cell lines but with lower potency than the extract. At a lethal dose, treatment with the extract or with usnic acid greatly increased the apoptotic cell population and specifically activated the apoptotic signaling pathway; however, using sub-lethal doses, extract and usnic acid treatment decreased cancer cell motility and inhibited in vitro and in vivo tumorigenic potentials. In these cells, we observed significantly reduced levels of epithelial-mesenchymal transition (EMT) markers and phosphor-Akt, while phosphor-c-Jun and phosphor-ERK1/2 levels were only marginally affected. Overall, the anti-cancer activity of the extract is more potent than that of usnic acid alone. Taken together, F. cucullata and its subcomponent, usnic acid together with additional component, exert anti-cancer effects on human cancer cells through the induction of apoptosis and the inhibition of EMT.
Conflict of interest statement
Figures


References
-
- WHO (2013) World Health Statistics 2013.
-
- Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63: 11–30. - PubMed
-
- Nash TH (2008) Lichen biology. Cambridge; New York: Cambridge University Press. ix, 486 p.
-
- Fashelt D (1994) Secondary biochemistry of lichens. Symbiosis 16: 117–165.
-
- Shrestha G, St. Clair LL (2013) Lichens: a promising source of antibiotic and anticancer drugs. Phytochem Rev 12: 229–244.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

