Wnt/β-catenin signaling modulates human airway sensitization induced by β2-adrenoceptor stimulation
- PMID: 25360795
- PMCID: PMC4216012
- DOI: 10.1371/journal.pone.0111350
Wnt/β-catenin signaling modulates human airway sensitization induced by β2-adrenoceptor stimulation
Abstract
Background: Regular use of β2-agonists may enhance non-specific airway responsiveness. The wingless/integrated (Wnt) signaling pathways are responsible for several cellular processes, including airway inflammation and remodeling while cAMP-PKA cascade can activate the Wnt signaling. We aimed to investigate whether the Wnt signaling pathways are involved in the bronchial hyperresponsiveness induced by prolonged exposure to β2-adrenoceptor agonists in human isolated airways.
Methods: Bronchi were surgically removed from 44 thoracic surgery patients. After preparation, bronchial rings and primary cultures of bronchial epithelial cells were incubated with fenoterol (0.1 µM, 15 hours, 37 °C), a β2-agonist with high intrinsic efficacy. The effects of inhibitors/blockers of Wnt signaling on the fenoterol-induced airway sensitization were examined and the impact of fenoterol exposure on the mRNA expression of genes interacting with Wnt signaling or cAMP-PKA cascade was assessed in complete bronchi and in cultured epithelial cells.
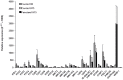
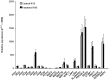
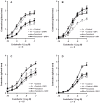
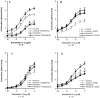
Results: Compared to paired controls, fenoterol-sensitization was abolished by inhibition/blockage of the Wnt/β-catenin signaling, especially the cell-surface LRP5/6 co-receptors or Fzd receptors (1 µM SFRP1 or 1 µM DKK1) and the nuclear recruitment of TCF/LEF transcriptions factors (0.3 µM FH535). Wnt proteins secretion did not seem to be involved in the fenoterol-induced sensitization since the mRNA expression of Wnt remained low after fenoterol exposure and the inactivator of Wnt secretion (1 µM IWP2) had no effect on the fenoterol-sensitization. Fenoterol exposure did not change the mRNA expression of genes regulating Wnt signaling or cAMP-PKA cascade.
Conclusions: Collectively, our pharmacological investigations indicate that fenoterol-sensitization is modulated by the inhibition/blockage of canonical Wnt/β-catenin pathway, suggesting a phenomenon of biased agonism in connection with the β2-adrenoceptor stimulation. Future experiments based on the results of the present study will be needed to determine the impact of prolonged fenoterol exposure on the extra- and intracellular Wnt signaling pathways at the protein expression level.
Conflict of interest statement
Figures
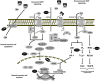
 and
and  , respectively. In the presence of Wnt ligand, Fzd and LRP5/6 form a receptor complex leading to the recruitment of cytosolic proteins Dsh and LRP5/6 phosphorylation. The phosphorylation and partial internalization of LRP5/6 initiate the disruption of the destruction complex (axin, APC, CK-1, and GSK-3β), allowing cytosolic β-catenin accumulation and then translocation into the nucleus, where β-catenin serves as a coactivator of TCF/LEF transcription factors to control gene transcription. Depending on the cellular context, the non-canonical Wnt signaling pathway is stimulated by the binding of Fzd and ROR2/RYK coreceptors. The Wnt/Ca2+ pathway induces PKC activation via the intracellular Ca2+ increase. The Wnt/PCP pathway activates the small G proteins Rho and the MAPK cascade via the recruitment of the cytosolic proteins Dsh and DAAM1, or, alternatively, triggers activation of JNK leading to the transcription of target genes through AP-1 activation.
, respectively. In the presence of Wnt ligand, Fzd and LRP5/6 form a receptor complex leading to the recruitment of cytosolic proteins Dsh and LRP5/6 phosphorylation. The phosphorylation and partial internalization of LRP5/6 initiate the disruption of the destruction complex (axin, APC, CK-1, and GSK-3β), allowing cytosolic β-catenin accumulation and then translocation into the nucleus, where β-catenin serves as a coactivator of TCF/LEF transcription factors to control gene transcription. Depending on the cellular context, the non-canonical Wnt signaling pathway is stimulated by the binding of Fzd and ROR2/RYK coreceptors. The Wnt/Ca2+ pathway induces PKC activation via the intracellular Ca2+ increase. The Wnt/PCP pathway activates the small G proteins Rho and the MAPK cascade via the recruitment of the cytosolic proteins Dsh and DAAM1, or, alternatively, triggers activation of JNK leading to the transcription of target genes through AP-1 activation.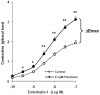
References
-
- Königshoff M, Eickelberg O (2010) WNT signaling in lung disease. A failure or a regeneration signal? Am J Resp Cell Mol Biol 42: 21–31. - PubMed
-
- Baron R, Kneissel M (2013) WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 19: 179–192. - PubMed
-
- Hahne G, Grossmann TN (2013) Direct targeting of β-catenin: inhibition of protein-protein interactions for the inactivation of Wnt signaling. Bioorg Med Chem 21: 4020–4026. - PubMed
-
- Baarsma HA, Königshoff M, Gosens R (2013) The WNT signaling pathway from ligand secretion to gene transcription: Molecular mechanisms and pharmacological targets. Pharmacol Ther 138: 66–83. - PubMed
-
- Reis M, Liebner S (2005) Wnt signaling in the vasculature. Exp Cell Res 319: 1317–1323. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

