Oncogenic BRAF induces chronic ER stress condition resulting in increased basal autophagy and apoptotic resistance of cutaneous melanoma
- PMID: 25361077
- PMCID: PMC4423179
- DOI: 10.1038/cdd.2014.183
Oncogenic BRAF induces chronic ER stress condition resulting in increased basal autophagy and apoptotic resistance of cutaneous melanoma
Abstract
The notorious unresponsiveness of metastatic cutaneous melanoma to current treatment strategies coupled with its increasing incidence constitutes a serious worldwide clinical problem. Moreover, despite recent advances in targeted therapies for patients with BRAF(V600E) mutant melanomas, acquired resistance remains a limiting factor and hence emphasises the acute need for comprehensive pre-clinical studies to increase the biological understanding of such tumours in order to develop novel effective and longlasting therapeutic strategies. Autophagy and ER stress both have a role in melanoma development/progression and chemoresistance although their real impact is still unclear. Here, we show that BRAF(V600E) induces a chronic ER stress status directly increasing basal cell autophagy. BRAF(V600E)-mediated p38 activation stimulates both the IRE1/ASK1/JNK and TRB3 pathways. Bcl-XL/Bcl-2 phosphorylation by active JNK releases Beclin1 whereas TRB3 inhibits the Akt/mTor axes, together resulting in an increase in basal autophagy. Furthermore, we demonstrate chemical chaperones relieve the BRAF(V600E)-mediated chronic ER stress status, consequently reducing basal autophagic activity and increasing the sensitivity of melanoma cells to apoptosis. Taken together, these results suggest enhanced basal autophagy, typically observed in BRAF(V600E) melanomas, is a consequence of a chronic ER stress status, which ultimately results in the chemoresistance of such tumours. Targeted therapies that attenuate ER stress may therefore represent a novel and more effective therapeutic strategy for BRAF mutant melanoma.
Figures


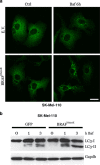


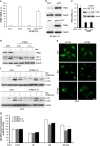

References
-
- Thompson JF, Scolyer RA, Kefford RF. Cutaneous melanoma. Lancet. 2005;365:687–701. - PubMed
-
- Cockerell CJ. The pathology of melanoma. Dermatol Clin. 2012;30:445–468. - PubMed
-
- Corazzari M, Fimia GM, Lovat P, Piacentini M. Why is autophagy important for melanoma? molecular mechanisms and therapeutic implications. Semin Cancer Biol. 2013;23:337–343. - PubMed
-
- Fecher LA, Cummings SD, Keefe MJ, Alani RM. Toward a molecular classification of melanoma. J Clin Oncol. 2007;25:1606–1620. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

