Role of Kv7 channels in responses of the pulmonary circulation to hypoxia
- PMID: 25361569
- PMCID: PMC4281702
- DOI: 10.1152/ajplung.00362.2013
Role of Kv7 channels in responses of the pulmonary circulation to hypoxia
Abstract
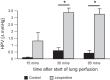
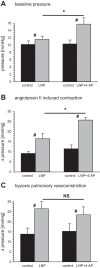
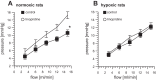
Hypoxic pulmonary vasoconstriction (HPV) is a beneficial mechanism that diverts blood from hypoxic alveoli to better ventilated areas of the lung, but breathing hypoxic air causes the pulmonary circulation to become hypertensive. Responses to airway hypoxia are associated with depolarization of smooth muscle cells in the pulmonary arteries and reduced activity of K(+) channels. As Kv7 channels have been proposed to play a key role in regulating the smooth muscle membrane potential, we investigated their involvement in the development of HPV and hypoxia-induced pulmonary hypertension. Vascular effects of the selective Kv7 blocker, linopirdine, and Kv7 activator, flupirtine, were investigated in isolated, saline-perfused lungs from rats maintained for 3-5 days in an isobaric hypoxic chamber (FiO2 = 0.1) or room air. Linopirdine increased vascular resistance in lungs from normoxic, but not hypoxic rats. This effect was associated with reduced mRNA expression of the Kv7.4 channel α-subunit in hypoxic arteries, whereas Kv7.1 and Kv7.5 were unaffected. Flupirtine had no effect in normoxic lungs but reduced vascular resistance in hypoxic lungs. Moreover, oral dosing with flupirtine (30 mg/kg/day) prevented short-term in vivo hypoxia from increasing pulmonary vascular resistance and sensitizing the arteries to acute hypoxia. These findings suggest a protective role for Kv7.4 channels in the pulmonary circulation, limiting its reactivity to pressor agents and preventing hypoxia-induced pulmonary hypertension. They also provide further support for the therapeutic potential of Kv7 activators in pulmonary vascular disease.
Keywords: KCNQ; Kv7 channels; P/Q relationship; flupirtine; hypoxic pulmonary vasoconstriction; isolated lungs.
Copyright © 2015 the American Physiological Society.
Figures


References
-
- Archer SL, Souil E, Dinh-Xuan AT, Schremmer B, Mercier JC, El Yaagoubi A, Nguyen-Huu L, Reeve HL, Hampl V. Molecular identification of the role of voltage-gated K+ channels, Kv1.5 and Kv21, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes. J Clin Invest 101: 2319–2330, 1998. - PMC - PubMed
-
- Archer SL, Weir EK, Reeve HL, Michelakis E. Molecular identification of O2 sensors and O2-sensitive potassium channels in the pulmonary circulation. Adv Exp Med Biol 475: 219–240, 2000. - PubMed
-
- Clapp LH, Gurney AM. ATP-sensitive K+ channels regulate resting potential of pulmonary arterial smooth muscle cells. Am J Physiol Heart Circ Physiol 262: H916–H920, 1992. - PubMed
-
- Clapp LH, Gurney AM. Modulation of calcium movements by nitroprusside in isolated vascular smooth muscle cells. Pflügers Arch 418: 462–470, 1991. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

