Cellulose surface degradation by a lytic polysaccharide monooxygenase and its effect on cellulase hydrolytic efficiency
- PMID: 25361767
- PMCID: PMC4276861
- DOI: 10.1074/jbc.M114.602227
Cellulose surface degradation by a lytic polysaccharide monooxygenase and its effect on cellulase hydrolytic efficiency
Abstract
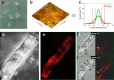
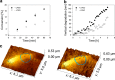
Lytic polysaccharide monooxygenase (LPMO) represents a unique principle of oxidative degradation of recalcitrant insoluble polysaccharides. Used in combination with hydrolytic enzymes, LPMO appears to constitute a significant factor of the efficiency of enzymatic biomass depolymerization. LPMO activity on different cellulose substrates has been shown from the slow release of oxidized oligosaccharides into solution, but an immediate and direct demonstration of the enzyme action on the cellulose surface is lacking. Specificity of LPMO for degrading ordered crystalline and unordered amorphous cellulose material of the substrate surface is also unknown. We show by fluorescence dye adsorption analyzed with confocal laser scanning microscopy that a LPMO (from Neurospora crassa) introduces carboxyl groups primarily in surface-exposed crystalline areas of the cellulosic substrate. Using time-resolved in situ atomic force microscopy we further demonstrate that cellulose nano-fibrils exposed on the surface are degraded into shorter and thinner insoluble fragments. Also using atomic force microscopy, we show that prior action of LPMO enables cellulases to attack otherwise highly resistant crystalline substrate areas and that it promotes an overall faster and more complete surface degradation. Overall, this study reveals key characteristics of LPMO action on the cellulose surface and suggests the effects of substrate morphology on the synergy between LPMO and hydrolytic enzymes in cellulose depolymerization.
Keywords: Atomic Force Microscopy (AFM); Biofuel; Cellulase; Cellulose; Copper Monooxygenase; GH61-AA9; Lytic Polysaccharide Monooxygenase (LPMO); Oxidative Cellulose Surface Degradation; Synergy.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Chundawat S. P., Beckham G. T., Himmel M. E., Dale B. E. (2011) Deconstruction of lignocellulosic biomass to fuels and chemicals. Annu. Rev. Chem. Biomol. Eng. 2, 121–145 - PubMed
-
- Kim I. J., Lee H. J., Choi I.-G., Kim K. H. (2014) Synergistic proteins for the enhanced enzymatic hydrolysis of cellulose by cellulase. Appl. Microbiol. Biotechnol. 98, 8469–8480 - PubMed
-
- Himmel M. E., Ding S.-Y., Johnson D. K., Adney W. S., Nimlos M. R., Brady J. W., Foust T. D. (2007) Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science 315, 804–807 - PubMed
-
- Harris P. V., Xu F., Kreel N. E., Kang C., Fukuyama S. (2014) New enzyme insights drive advances in commercial ethanol production. Curr. Opin. Chem. Biol. 19, 162–170 - PubMed
-
- Hu J., Arantes V., Pribowo A., Gourlay K., Saddler J. N. (2014) Substrate factors that influence the synergistic interaction of AA9 and cellulases during the enzymatic hydrolysis of biomass. Energy Environ. Sci. 7, 2308–2315
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

