Phyllosphere bacterial community of floating macrophytes in paddy soil environments as revealed by illumina high-throughput sequencing
- PMID: 25362067
- PMCID: PMC4277568
- DOI: 10.1128/AEM.03191-14
Phyllosphere bacterial community of floating macrophytes in paddy soil environments as revealed by illumina high-throughput sequencing
Abstract
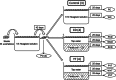
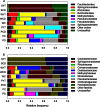
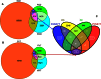
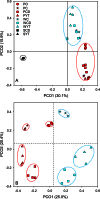
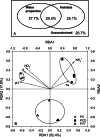
The phyllosphere of floating macrophytes in paddy soil ecosystems, a unique habitat, may support large microbial communities but remains largely unknown. We took Wolffia australiana as a representative floating plant and investigated its phyllosphere bacterial community and the underlying driving forces of community modulation in paddy soil ecosystems using Illumina HiSeq 2000 platform-based 16S rRNA gene sequence analysis. The results showed that the phyllosphere of W. australiana harbored considerably rich communities of bacteria, with Proteobacteria and Bacteroidetes as the predominant phyla. The core microbiome in the phyllosphere contained genera such as Acidovorax, Asticcacaulis, Methylibium, and Methylophilus. Complexity of the phyllosphere bacterial communities in terms of class number and α-diversity was reduced compared to those in corresponding water and soil. Furthermore, the bacterial communities exhibited structures significantly different from those in water and soil. These findings and the following redundancy analysis (RDA) suggest that species sorting played an important role in the recruitment of bacterial species in the phyllosphere. The compositional structures of the phyllosphere bacterial communities were modulated predominantly by water physicochemical properties, while the initial soil bacterial communities had limited impact. Taken together, the findings from this study reveal the diversity and uniqueness of the phyllosphere bacterial communities associated with the floating macrophytes in paddy soil environments.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

