Compartmentalization of simian immunodeficiency virus replication within secondary lymphoid tissues of rhesus macaques is linked to disease stage and inversely related to localization of virus-specific CTL
- PMID: 25362178
- PMCID: PMC4239212
- DOI: 10.4049/jimmunol.1401161
Compartmentalization of simian immunodeficiency virus replication within secondary lymphoid tissues of rhesus macaques is linked to disease stage and inversely related to localization of virus-specific CTL
Abstract
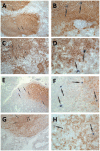
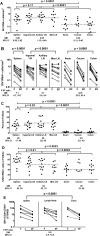
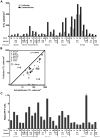
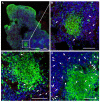
We previously demonstrated that HIV replication is concentrated in lymph node B cell follicles during chronic infection and that HIV-specific CTL fail to accumulate in large numbers at those sites. It is unknown whether these observations can be generalized to other secondary lymphoid tissues or whether virus compartmentalization occurs in the absence of CTL. We evaluated these questions in SIVmac239-infected rhesus macaques by quantifying SIV RNA(+) cells and SIV-specific CTL in situ in spleen, lymph nodes, and intestinal tissues obtained at several stages of infection. During chronic asymptomatic infection prior to simian AIDS, SIV-producing cells were more concentrated in follicular (F) compared with extrafollicular (EF) regions of secondary lymphoid tissues. At day 14 of infection, when CTL have minimal impact on virus replication, there was no compartmentalization of SIV-producing cells. Virus compartmentalization was diminished in animals with simian AIDS, which often have low-frequency CTL responses. SIV-specific CTL were consistently more concentrated within EF regions of lymph node and spleen in chronically infected animals regardless of epitope specificity. Frequencies of SIV-specific CTL within F and EF compartments predicted SIV RNA(+) cells within these compartments in a mixed model. Few SIV-specific CTL expressed the F homing molecule CXCR5 in the absence of the EF retention molecule CCR7, possibly accounting for the paucity of F CTL. These findings bolster the hypothesis that B cell follicles are immune privileged sites and suggest that strategies to augment CTL in B cell follicles could lead to improved viral control and possibly a functional cure for HIV infection.
Copyright © 2014 by The American Association of Immunologists, Inc.
Figures


References
-
- Connick E, Mattila T, Folkvord JM, Schlichtemeier R, Meditz AL, Ray MG, McCarter MD, Mawhinney S, Hage A, White C, Skinner PJ. CTL fail to accumulate at sites of HIV-1 replication in lymphoid tissue. J Immunol. 2007;178:6975–6983. - PubMed
-
- Folkvord JM, Armon C, Connick E. Lymphoid follicles are sites of heightened human immunodeficiency virus type 1 (HIV-1) replication and reduced antiretroviral effector mechanisms. AIDS Res Hum Retroviruses. 2005;21:363–370. - PubMed
-
- Hufert FT, van Lunzen J, Janossy G, Bertram S, Schmitz J, Haller O, Racz P, von Laer D. Germinal centre CD4+ T cells are an important site of HIV replication in vivo. Aids. 1997;11:849–857. - PubMed
-
- Tenner-Racz K, Stellbrink HJ, van Lunzen J, Schneider C, Jacobs JP, Raschdorff B, Grosschupff G, Steinman RM, Racz P. The unenlarged lymph nodes of HIV-1-infected, asymptomatic patients with high CD4 T cell counts are sites for virus replication and CD4 T cell proliferation. The impact of highly active antiretroviral therapy. J Exp Med. 1998;187:949–959. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI090795/AI/NIAID NIH HHS/United States
- P51 RR000167/RR/NCRR NIH HHS/United States
- R01 AI096966/AI/NIAID NIH HHS/United States
- T32 AI007447/AI/NIAID NIH HHS/United States
- R56AI080418/AI/NIAID NIH HHS/United States
- R01AI090795/AI/NIAID NIH HHS/United States
- HHSN272201300006C/AI/NIAID NIH HHS/United States
- U24 AI126683/AI/NIAID NIH HHS/United States
- P51RR000167/RR/NCRR NIH HHS/United States
- R56 AI080418/AI/NIAID NIH HHS/United States
- HHSN272200900037C/AI/NIAID NIH HHS/United States
- R24 RR016001/RR/NCRR NIH HHS/United States
- P51 OD011106/OD/NIH HHS/United States
- P51OD011106/OD/NIH HHS/United States
- R01AI096966/AI/NIAID NIH HHS/United States
- HHSN 2722000900037C/PHS HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

