Glial ankyrins facilitate paranodal axoglial junction assembly
- PMID: 25362471
- PMCID: PMC4260775
- DOI: 10.1038/nn.3858
Glial ankyrins facilitate paranodal axoglial junction assembly
Abstract
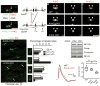
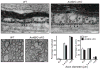
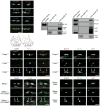
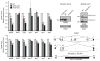
Neuron-glia interactions establish functional membrane domains along myelinated axons. These include nodes of Ranvier, paranodal axoglial junctions and juxtaparanodes. Paranodal junctions are the largest vertebrate junctional adhesion complex, and they are essential for rapid saltatory conduction and contribute to assembly and maintenance of nodes. However, the molecular mechanisms underlying paranodal junction assembly are poorly understood. Ankyrins are cytoskeletal scaffolds traditionally associated with Na(+) channel clustering in neurons and are important for membrane domain establishment and maintenance in many cell types. Here we show that ankyrin-B, expressed by Schwann cells, and ankyrin-G, expressed by oligodendrocytes, are highly enriched at the glial side of paranodal junctions where they interact with the essential glial junctional component neurofascin 155. Conditional knockout of ankyrins in oligodendrocytes disrupts paranodal junction assembly and delays nerve conduction during early development in mice. Thus, glial ankyrins function as major scaffolds that facilitate early and efficient paranodal junction assembly in the developing CNS.
Conflict of interest statement
Competing Financial Interests:
The authors declare no competing financial interests.
Figures




References
-
- Poliak S, Peles E. The local differentiation of myelinated axons at nodes of Ranvier. Nat Rev Neurosci. 2003;4:968–980. - PubMed
-
- Salzer JL. Polarized domains of myelinated axons. Neuron. 2003;40:297–318. - PubMed
-
- Chang KJ, Rasband MN. Excitable domains of myelinated nerves: axon initial segments and nodes of Ranvier. Curr Top Membr. 2013;72:159–192. - PubMed
-
- Bennett V, Lorenzo DN. Spectrin- and ankyrin-based membrane domains and the evolution of vertebrates. Curr Top Membr. 2013;72:1–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL114383/HL/NHLBI NIH HHS/United States
- U54 HD083092/HD/NICHD NIH HHS/United States
- R01 NS049119/NS/NINDS NIH HHS/United States
- HL084583,/HL/NHLBI NIH HHS/United States
- NS069688/NS/NINDS NIH HHS/United States
- NS044916/NS/NINDS NIH HHS/United States
- HL114383/HL/NHLBI NIH HHS/United States
- R37 NS044916/NS/NINDS NIH HHS/United States
- R01 NS044916/NS/NINDS NIH HHS/United States
- R01 HL083422/HL/NHLBI NIH HHS/United States
- HL083422/HL/NHLBI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 HL084583/HL/NHLBI NIH HHS/United States
- MR/L011379/1/MRC_/Medical Research Council/United Kingdom
- R01 NS069688/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

