Autophagy is essential for effector CD8(+) T cell survival and memory formation
- PMID: 25362489
- PMCID: PMC4232981
- DOI: 10.1038/ni.3025
Autophagy is essential for effector CD8(+) T cell survival and memory formation
Abstract
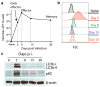
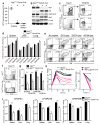
The importance of autophagy in the generation of memory CD8(+) T cells in vivo is not well defined. We report here that autophagy was dynamically regulated in virus-specific CD8(+) T cells during acute infection of mice with lymphocytic choriomeningitis virus. In contrast to the current paradigm, autophagy decreased in activated proliferating effector CD8(+) T cells and was then upregulated when the cells stopped dividing just before the contraction phase. Consistent with those findings, deletion of the gene encoding either of the autophagy-related molecules Atg5 or Atg7 had little to no effect on the proliferation and function of effector cells, but these autophagy-deficient effector cells had survival defects that resulted in compromised formation of memory T cells. Our studies define when autophagy is needed during effector and memory differentiation and warrant reexamination of the relationship between T cell activation and autophagy.
Conflict of interest statement
The authors declare no competing financial interests.
Figures






Comment in
-
Cell death: breaking down memory.Nat Rev Immunol. 2014 Dec;14(12):778-9. doi: 10.1038/nri3774. Epub 2014 Nov 14. Nat Rev Immunol. 2014. PMID: 25394943 No abstract available.
References
-
- Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. - PubMed
-
- Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4(12):1191–1198. - PubMed
Publication types
MeSH terms
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

