Making microvascular networks work: angiogenesis, remodeling, and pruning
- PMID: 25362638
- PMCID: PMC4280154
- DOI: 10.1152/physiol.00012.2014
Making microvascular networks work: angiogenesis, remodeling, and pruning
Abstract
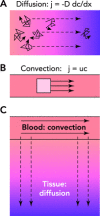
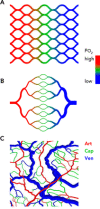
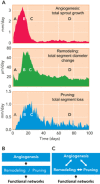
The adequate and efficient functioning of the microcirculation requires not only numerous vessels providing a large surface area for transport but also a structure that provides short diffusion distances from capillaries to tissue and efficient distribution of convective blood flow. Theoretical models show how a combination of angiogenesis, remodeling, and pruning in response to hemodynamic and metabolic stimuli, termed "angioadaptation," generates well organized, functional networks.
©2014 Int. Union Physiol. Sci./Am. Physiol. Soc.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the author(s).
Figures



References
-
- Ahmad S, Hewett PW, Wang P, Al-Ani B, Cudmore M, Fujisawa T, Haigh JJ, Le Noble F, Wang L, Mukhopadhyay D, Ahmed A. Direct evidence for endothelial vascular endothelial growth factor receptor-1 function in nitric oxide-mediated angiogenesis. Circ Res 99: 715–722, 2006. - PubMed
-
- Anderson AR, Chaplain MA. Continuous and discrete mathematical models of tumor-induced angiogenesis. Bull Math Biol 60: 857–899, 1998. - PubMed
-
- Andres AC, Djonov V. The mammary gland vasculature revisited. J Mammary Gland Biol Neoplasia 15: 319–328, 2010. - PubMed
-
- Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol 10: 165–177, 2009. - PubMed
-
- Baish JW, Jain RK. Cancer, angiogenesis and fractals. Nat Med 4: 984, 1998. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

