Synthesis and SAR studies of novel 6,7,8-substituted 4-substituted benzyloxyquinolin-2(1H)-one derivatives for anticancer activity
- PMID: 25363404
- PMCID: PMC4337696
- DOI: 10.1111/bph.12992
Synthesis and SAR studies of novel 6,7,8-substituted 4-substituted benzyloxyquinolin-2(1H)-one derivatives for anticancer activity
Abstract
Background and purpose: 4-Phenylquinolin-2(1H)-one (4-PQ) derivatives can induce cancer cell apoptosis. Additional new 4-PQ analogs were investigated as more effective, less toxic antitumour agents.
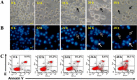
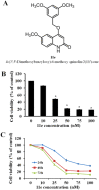
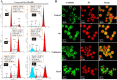
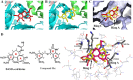
Experimental approach: Forty-five 6,7,8-substituted 4-substituted benzyloxyquinolin-2(1H)-one derivatives were synthesized. Antiproliferative activities were evaluated using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliun bromide assay and structure-activity relationship correlations were established. Compounds 9b, 9c, 9e and 11e were also evaluated against the National Cancer Institute-60 human cancer cell line panel. Hoechst 33258 and Annexin V-FITC/PI staining assays were used to detect apoptosis, while inhibition of microtubule polymerization was assayed by fluorescence microscopy. Effects on the cell cycle were assessed by flow cytometry and on apoptosis-related proteins (active caspase-3, -8 and -9, procaspase-3, -8, -9, PARP, Bid, Bcl-xL and Bcl-2) by Western blotting.
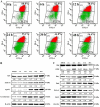
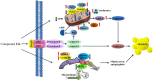
Key results: Nine 6,7,8-substituted 4-substituted benzyloxyquinolin-2(1H)-one derivatives (7e, 8e, 9b, 9c, 9e, 10c, 10e, 11c and 11e) displayed high potency against HL-60, Hep3B, H460, and COLO 205 cancer cells (IC₅₀ < 1 μM) without affecting Detroit 551 normal human cells (IC₅₀ > 50 μM). Particularly, compound 11e exhibited nanomolar potency against COLO 205 cancer cells. Mechanistic studies indicated that compound 11e disrupted microtubule assembly and induced G2/M arrest, polyploidy and apoptosis via the intrinsic and extrinsic signalling pathways. Activation of JNK could play a role in TRAIL-induced COLO 205 apoptosis.
Conclusion and implications: New quinolone derivatives were identified as potential pro-apoptotic agents. Compound 11e could be a promising lead compound for future antitumour agent development.
© 2014 The British Pharmacological Society.
Figures









References
-
- Abonia R, Insuasty D, Castillo J, Insuasty B, Quiroga J, Nogueras M, et al. Synthesis of novel quinoline-2-one based chalcones of potential anti-tumor activity. Eur J Med Chem. 2012;57:29–40. - PubMed
-
- Ahmed N, Brahmbhatt KG, Sabde S, Mitra D, Singh IP, Bhutani KK. Synthesis and anti-HIV activity of alkylated quinoline 2,4-diols. Bioorg Med Chem. 2010;18:2872–2879. - PubMed
-
- Ahmed N, Brahmbhatt KG, Singh IP, Bhutani KK. Efficient chemoselective alkylation of quinoline 2,4-diol derivatives in water. J Heterocycl Chem. 2011;48:237–240.
-
- Ahvale AB, Prokopcová H, Šefčovičová J, Steinschifter W, Täubl AE, Uray G, et al. 4-Cyano-6,7-dimethoxycarbostyrils with solvent- and pH-independent high fluorescence quantum yields and emission maxima. Eur J Org Chem. 2008;2008:563–571.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

