Primaquine: the risks and the benefits
- PMID: 25363455
- PMCID: PMC4230503
- DOI: 10.1186/1475-2875-13-418
Primaquine: the risks and the benefits
Abstract
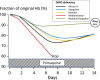
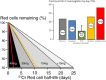
Primaquine is the only generally available anti-malarial that prevents relapse in vivax and ovale malaria, and the only potent gametocytocide in falciparum malaria. Primaquine becomes increasingly important as malaria-endemic countries move towards elimination, and although it is widely recommended, it is commonly not given to malaria patients because of haemolytic toxicity in subjects who are glucose-6-phosphate dehydrogenase (G6PD) deficient (gene frequency typically 3-30% in malaria endemic areas; >180 different genetic variants). In six decades of primaquine use in approximately 200 million people, 14 deaths have been reported. Confining the estimate to reports with known denominators gives an estimated mortality of one in 621,428 (upper 95% CI: one in 407,807). All but one death followed multiple dosing to prevent vivax malaria relapse. Review of dose-response relationships and clinical trials of primaquine in G6PD deficiency suggests that the currently recommended WHO single low dose (0.25 mg base/kg) to block falciparum malaria transmission confers a very low risk of haemolytic toxicity.
Figures
References
-
- Recht J, Ashley EA, White NJ. Safety of 8-Aminoquinoline Antimalarial Medicines. Geneva: World Health Organization; 2014.
-
- Alving AS, Johnson CF, Tarlov AR, Brewer GJ, Kellermeyer RW, Carson PE. Mitigation of the haemolytic effect of primaquine and enhancement of its action against exoerythrocytic forms of the Chesson strain of Plasmodium vivax by intermittent regimens of drug administration: a preliminary report. Bull World Health Organ. 1960;22:621–631. - PMC - PubMed
-
- Hsiang MS, Hwang J, Tao AR, Liu Y, Bennett A, Shanks GD, Cao J, Kachur SP, Feachem RG, Gosling RD, Gao Q. Mass drug administration for the control and elimination of Plasmodium vivax malaria: an ecological study from Jiangsu province. China Malar J. 2013;12:383. doi: 10.1186/1475-2875-12-383. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

