The virtual heart as a platform for screening drug cardiotoxicity
- PMID: 25363597
- PMCID: PMC4667856
- DOI: 10.1111/bph.12996
The virtual heart as a platform for screening drug cardiotoxicity
Abstract
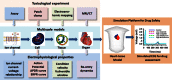
To predict the safety of a drug at an early stage in its development is a major challenge as there is a lack of in vitro heart models that correlate data from preclinical toxicity screening assays with clinical results. A biophysically detailed computer model of the heart, the virtual heart, provides a powerful tool for simulating drug-ion channel interactions and cardiac functions during normal and disease conditions and, therefore, provides a powerful platform for drug cardiotoxicity screening. In this article, we first review recent progress in the development of theory on drug-ion channel interactions and mathematical modelling. Then we propose a family of biomarkers that can quantitatively characterize the actions of a drug on the electrical activity of the heart at multi-physical scales including cellular and tissue levels. We also conducted some simulations to demonstrate the application of the virtual heart to assess the pro-arrhythmic effects of cisapride and amiodarone. Using the model we investigated the mechanisms responsible for the differences between the two drugs on pro-arrhythmogenesis, even though both prolong the QT interval of ECGs. Several challenges for further development of a virtual heart as a platform for screening drug cardiotoxicity are discussed.
© 2014 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of The British Pharmacological Society.
Figures









References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

