Modelling human development and disease in pluripotent stem-cell-derived gastric organoids
- PMID: 25363776
- PMCID: PMC4270898
- DOI: 10.1038/nature13863
Modelling human development and disease in pluripotent stem-cell-derived gastric organoids
Abstract
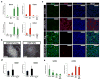
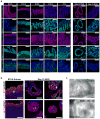
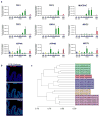
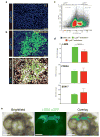
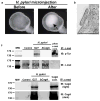
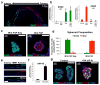
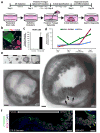
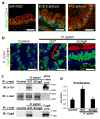
Gastric diseases, including peptic ulcer disease and gastric cancer, affect 10% of the world's population and are largely due to chronic Helicobacter pylori infection. Species differences in embryonic development and architecture of the adult stomach make animal models suboptimal for studying human stomach organogenesis and pathogenesis, and there is no experimental model of normal human gastric mucosa. Here we report the de novo generation of three-dimensional human gastric tissue in vitro through the directed differentiation of human pluripotent stem cells. We show that temporal manipulation of the FGF, WNT, BMP, retinoic acid and EGF signalling pathways and three-dimensional growth are sufficient to generate human gastric organoids (hGOs). Developing hGOs progressed through molecular and morphogenetic stages that were nearly identical to the developing antrum of the mouse stomach. Organoids formed primitive gastric gland- and pit-like domains, proliferative zones containing LGR5-expressing cells, surface and antral mucous cells, and a diversity of gastric endocrine cells. We used hGO cultures to identify novel signalling mechanisms that regulate early endoderm patterning and gastric endocrine cell differentiation upstream of the transcription factor NEUROG3. Using hGOs to model pathogenesis of human disease, we found that H. pylori infection resulted in rapid association of the virulence factor CagA with the c-Met receptor, activation of signalling and induction of epithelial proliferation. Together, these studies describe a new and robust in vitro system for elucidating the mechanisms underlying human stomach development and disease.
Figures






References
Publication types
MeSH terms
Grants and funding
- K01DK091415/DK/NIDDK NIH HHS/United States
- P30 DK078392/DK/NIDDK NIH HHS/United States
- 5P30DK034933/DK/NIDDK NIH HHS/United States
- T32 GM063483/GM/NIGMS NIH HHS/United States
- R01DK092456/DK/NIDDK NIH HHS/United States
- U19 AI116491/AI/NIAID NIH HHS/United States
- R01 DK080823/DK/NIDDK NIH HHS/United States
- R01DK080823/DK/NIDDK NIH HHS/United States
- K01 DK091415/DK/NIDDK NIH HHS/United States
- U54 RR025216/RR/NCRR NIH HHS/United States
- UL1 RR026314/RR/NCRR NIH HHS/United States
- P30 DK0789392/DK/NIDDK NIH HHS/United States
- R01 GM072915/GM/NIGMS NIH HHS/United States
- R01 DK092456/DK/NIDDK NIH HHS/United States
- P30 DK034933/DK/NIDDK NIH HHS/United States
- R01 DK098350/DK/NIDDK NIH HHS/United States
- R01 DK083402/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

