Neuroimaging biomarkers for Parkinson disease: facts and fantasy
- PMID: 25363872
- PMCID: PMC4245400
- DOI: 10.1002/ana.24291
Neuroimaging biomarkers for Parkinson disease: facts and fantasy
Abstract
In this grand rounds, we focus on development, validation, and application of neuroimaging biomarkers for Parkinson disease (PD). We cover whether such biomarkers can be used to identify presymptomatic individuals (probably yes), provide a measure of PD severity (in a limited fashion, but frequently done poorly), investigate pathophysiology of parkinsonian disorders (yes, if done carefully), play a role in differential diagnosis of parkinsonism (not well), and investigate pathology underlying cognitive impairment (yes, in conjunction with postmortem data). Along the way, we clarify several issues about definitions of biomarkers and surrogate endpoints. The goal of this lecture is to provide a basis for interpreting current literature and newly proposed clinical tools in PD. In the end, one should be able to critically distinguish fact from fantasy.
© 2014 American Neurological Association.
Figures

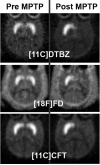

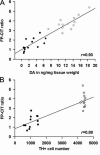
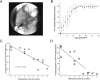
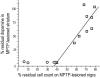

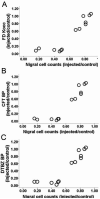
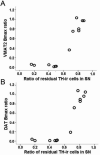
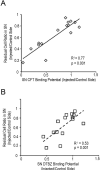
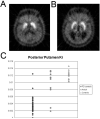
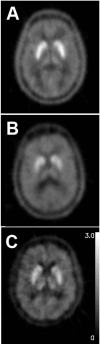

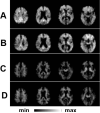
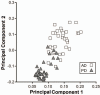
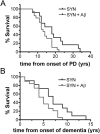
References
-
- Brooks DJ, Frey KA, Marek KL, et al. Assessment of neuroimaging techniques as biomarkers of the progression of Parkinson's disease. Exp Neurol. 2003;184(Suppl 1):S68–S79. - PubMed
-
- Perlmutter JS, Tempel LW, Black KJ, et al. MPTP induces dystonia and parkinsonism: Clues to the pathophysiology of dystonia. Neurology. 1997;49:1432–1438. - PubMed
-
- Burn DJ, Mark MH, Playford ED, et al. Parkinson's disease in twins studied with 18F-dopa and positron emission tomography. Neurology. 1992;42:1894–1900. - PubMed
-
- Nagai Y, Obayashi S, Ando K, et al. Progressive changes of pre- and post-synaptic dopaminergic biomarkers in conscious MPTP-treated cynomolgus monkeys measured by positron emission tomography. Synapse. 2007;61:809–819. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

