Ginger components as new leads for the design and development of novel multi-targeted anti-Alzheimer's drugs: a computational investigation
- PMID: 25364231
- PMCID: PMC4211852
- DOI: 10.2147/DDDT.S67778
Ginger components as new leads for the design and development of novel multi-targeted anti-Alzheimer's drugs: a computational investigation
Abstract
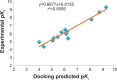
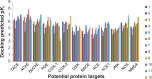
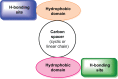
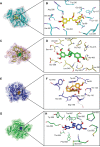
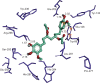
Ginger (Zingiber officinale), despite being a common dietary adjunct that contributes to the taste and flavor of foods, is well known to contain a number of potentially bioactive phytochemicals having valuable medicinal properties. Although recent studies have emphasized their benefits in Alzheimer's disease, limited information is available on the possible mechanism by which it renders anti-Alzheimer activity. Therefore, the present study seeks to employ molecular docking studies to investigate the binding interactions between active ginger components and various anti-Alzheimer drug targets. Lamarckian genetic algorithm methodology was employed for docking of 12 ligands with 13 different target proteins using AutoDock 4.2 program. Docking protocol was validated by re-docking of all native co-crystallized ligands into their original binding cavities exhibiting a strong correlation coefficient value (r (2)=0.931) between experimentally reported and docking predicted activities. This value suggests that the approach could be a promising computational tool to aid optimization of lead compounds obtained from ginger. Analysis of binding energy, predicted inhibition constant, and hydrophobic/hydrophilic interactions of ligands with target receptors revealed acetylcholinesterase as most promising, while c-Jun N-terminal kinase was recognized as the least favorable anti-Alzheimer's drug target. Common structural requirements include hydrogen bond donor/acceptor area, hydrophobic domain, carbon spacer, and distal hydrophobic domain flanked by hydrogen bond donor/acceptor moieties. In addition, drug-likeness score and molecular properties responsible for a good pharmacokinetic profile were calculated by Osiris property explorer and Molinspiration online toolkit, respectively. None of the compounds violated Lipinski's rule of five, making them potentially promising drug candidates for the treatment of Alzheimer's disease.
Keywords: Alzheimer’s disease; ginger; molecular docking; structure–activity relationship; toxicity prediction.
Figures



References
-
- Azam F. Therapeutic potential of free radical scavengers in neurological disorders. In: Kozyrev D, Slutsky V, editors. Handbook of Free radicals: Formation, Types and Effects. New York: Nova Publishers; 2010. pp. 57–97.
-
- Mandel S, Youdim MB. Catechin polyphenols: neurodegeneration and neuroprotection in neurodegenerative diseases. Free Radic Biol Med. 2004;37(3):304–317. - PubMed
-
- Chancellor B, Duncan A, Chatterjee A. Art therapy for Alzheimer’s disease and other dementias. J Alzheimers Dis. 2014;39(1):1–11. - PubMed
-
- Allain H, Bentue-Ferrer D, Tribut O, Gauthier S, Michel BF, Drieu-La Rochelle C. Alzheimer’s disease: the pharmacological pathway. Fundam Clin Pharmacol. 2003;17(4):419–428. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

