Strategies for gene disruption in Drosophila
- PMID: 25364499
- PMCID: PMC4216337
- DOI: 10.1186/2045-3701-4-63
Strategies for gene disruption in Drosophila
Abstract
Drosophila melanogaster has been a classic model organism for the studies of genetics. More than 15,000 Drosophila genes have been annotated since the entire genome was sequenced; however, many of them still lack functional characterization. Various gene-manipulating approaches in Drosophila have been developed for the function analysis of genes. Here, we summarize some representative strategies utilized for Drosophila gene targeting, from the unbiased ethyl methanesulfonate (EMS) mutagenesis and transposable element insertion, to insertional/replacement homologous recombination and site-specific nucleases such as the zinc-finger nuclease (ZFN), the transcription activator-like effector nuclease (TALEN) and the CRISPR (clustered regularly interspaced short palindromic repeats)/Cas9 system. Specifically, we evaluate the pros and cons of each technique in a historical perspective. This review discuss important factors that should be taken into consideration for the selection of a strategy that best fits the specific needs of a gene knockout project.
Keywords: CRISPR; Cas9; Drosophila; Gene knock-out; Genome editing; Homologous recombination; Transposons.
Figures
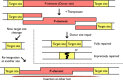
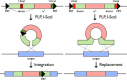
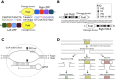
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

