Fibroblast growth factor 21 improves insulin sensitivity and synergizes with insulin in human adipose stem cell-derived (hASC) adipocytes
- PMID: 25365322
- PMCID: PMC4218812
- DOI: 10.1371/journal.pone.0111767
Fibroblast growth factor 21 improves insulin sensitivity and synergizes with insulin in human adipose stem cell-derived (hASC) adipocytes
Abstract
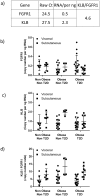
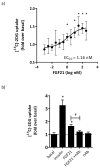
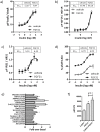
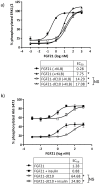
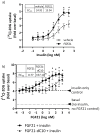
Fibroblast growth factor 21 (FGF21) has evolved as a major metabolic regulator, the pharmacological administration of which causes weight loss, insulin sensitivity and glucose control in rodents and humans. To understand the molecular mechanisms by which FGF21 exerts its metabolic effects, we developed a human in vitro model of adipocytes to examine crosstalk between FGF21 and insulin signaling. Human adipose stem cell-derived (hASC) adipocytes were acutely treated with FGF21 alone, insulin alone, or in combination. Insulin signaling under these conditions was assessed by measuring tyrosine phosphorylation of insulin receptor (InsR), insulin receptor substrate-1 (IRS-1), and serine 473 phosphorylation of Akt, followed by a functional assay using 14C-2-deoxyglucose [14C]-2DG to measure glucose uptake in these cells. FGF21 alone caused a modest increase of glucose uptake, but treatment with FGF21 in combination with insulin had a synergistic effect on glucose uptake in these cells. The presence of FGF21 also effectively lowered the insulin concentration required to achieve the same level of glucose uptake compared to the absence of FGF21 by 10-fold. This acute effect of FGF21 on insulin signaling was not due to IR, IGF-1R, or IRS-1 activation. Moreover, we observed a substantial increase in basal S473-Akt phosphorylation by FGF21 alone, in contrast to the minimal shift in basal glucose uptake. Taken together, our data demonstrate that acute co-treatment of hASC-adipocytes with FGF21 and insulin can result in a synergistic improvement in glucose uptake. These effects were shown to occur at or downstream of Akt, or separate from the canonical insulin signaling pathway.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

