Bioartificial heart: a human-sized porcine model--the way ahead
- PMID: 25365554
- PMCID: PMC4218780
- DOI: 10.1371/journal.pone.0111591
Bioartificial heart: a human-sized porcine model--the way ahead
Abstract
Background: A bioartificial heart is a theoretical alternative to transplantation or mechanical left ventricular support. Native hearts decellularized with preserved architecture and vasculature may provide an acellular tissue platform for organ regeneration. We sought to develop a tissue-engineered whole-heart neoscaffold in human-sized porcine hearts.
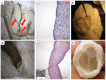
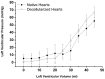
Methods: We decellularized porcine hearts (n = 10) by coronary perfusion with ionic detergents in a modified Langendorff circuit. We confirmed decellularization by histology, transmission electron microscopy and fluorescence microscopy, quantified residual DNA by spectrophotometry, and evaluated biomechanical stability with ex-vivo left-ventricular pressure/volume studies, all compared to controls. We then mounted the decellularized porcine hearts in a bioreactor and reseeded them with murine neonatal cardiac cells and human umbilical cord derived endothelial cells (HUVEC) under simulated physiological conditions.
Results: Decellularized hearts lacked intracellular components but retained specific collagen fibers, proteoglycan, elastin and mechanical integrity; quantitative DNA analysis demonstrated a significant reduction of DNA compared to controls (82.6±3.2 ng DNA/mg tissue vs. 473.2±13.4 ng DNA/mg tissue, p<0.05). Recellularized porcine whole-heart neoscaffolds demonstrated re-endothelialization of coronary vasculature and measurable intrinsic myocardial electrical activity at 10 days, with perfused organ culture maintained for up to 3 weeks.
Conclusions: Human-sized decellularized porcine hearts provide a promising tissue-engineering platform that may lead to future clinical strategies in the treatment of heart failure.
Conflict of interest statement
Figures





References
-
- Kobashigawa JA, Patel JK (2006) Immunosuppression for heart transplantation: Where are we now? Nat Clin Pract Cardiovasc Med 3: 203–212. - PubMed
-
- Badylak SF, Freytes DO, Gilbert TW (2009) Extracellular matrix as a biological scaffold material: Structure and function. Acta biomaterialia 5: 1–13. - PubMed
-
- Weymann A, Dohmen PM, Grubitzsch H, Dushe S, Holinski S, et al. (2010) Clinical experience with expanded use of the Ross procedure: A paradigm shift? J Heart Valve Dis 19: 279–285. - PubMed
-
- Weymann A, Radovits T, Schmack B, Li S, Korkmaz S, et al. (2014) In vitro generation of atrioventricular heart valve neoscaffolds. Artif Organs 38: 118–128. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

