Plasmon-enhanced optical sensors: a review
- PMID: 25365823
- PMCID: PMC4274271
- DOI: 10.1039/c4an01079e
Plasmon-enhanced optical sensors: a review
Abstract
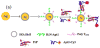
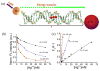
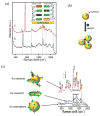
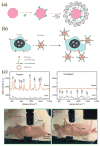
Surface plasmon resonance (SPR) has found extensive applications in chemi-sensors and biosensors. Plasmons play different roles in different types of optical sensors. SPR transduces a signal in a colorimetric sensor through shifts in the spectral position and intensity in response to external stimuli. SPR can also concentrate the incident electromagnetic field in a nanostructure, modulating fluorescence emission and enabling plasmon-enhanced fluorescence to be used for ultrasensitive detection. Furthermore, plasmons have been extensively used for amplifying a Raman signal in a surface-enhanced Raman scattering sensor. This paper presents a review of recent research progress in plasmon-enhanced optical sensing, giving emphasis on the physical basis of plasmon-enhanced sensors and how these principles guide the design of sensors. In particular, this paper discusses the design strategies for nanomaterials and nanostructures to plasmonically enhance optical sensing signals, also highlighting the applications of plasmon-enhanced optical sensors in healthcare, homeland security, food safety and environmental monitoring.
Figures









References
-
- Jones MR, Osberg KD, Macfarlane RJ, Langille MR, Mirkin CA. Chem Rev. 2011;111:3736–3827. - PubMed
-
- Ringe E, Zhang J, Langille MR, Sohn K, Cobley C, Au L, Xia Y, Mirkin CA, Huang J, Marks LD, Van Duyne RP. Mater Res Soc Symp Proc. 2010;1208:O10–02.
-
- Ringe E, Langille MR, Sohn K, Zhang J, Huang J, Mirkin CA, Van Duyne RP, Marks LD. J Phys Chem Lett. 2012;3:1479–1483. - PubMed
-
- Jain PK, El-Sayed MA. J Phys Chem C. 2007;111:17451–17454.
-
- Brandl DW, Mirin NA, Nordlander P. J Phys Chem B. 2006;110:12302–12310. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

