In vitro molecular evolution yields an NEIBM with a potential novel IgG binding property
- PMID: 25366194
- PMCID: PMC4219159
- DOI: 10.1038/srep06908
In vitro molecular evolution yields an NEIBM with a potential novel IgG binding property
Abstract
Staphylococcus aureus protein A (SpA) and protein G of groups C and G streptococci (SpG) are two well-defined bacterial immunoglobulin (Ig)-binding proteins (IBPs) with high affinity for specific sites on IgG from mammalian hosts. Both SpA and SpG contain several highly-homologous IgG-binding domains, each of which possesses similar binding characteristic of the whole corresponding proteins. Whether specific combinations of these domains could generate a molecule with novel IgG-binding properties remained unknown. We constructed a combinatorial phage library displaying randomly-rearranged A, B, C, D and E domains of SpA as well as the B2 (G2) and B3 (G3) domains of SpG. In vitro molecular evolution directed by human, rabbit, bovine, or goat polyclonal IgGs and four subclasses of mouse monoclonal IgGs generated one common combination, D-C-G3. A series of assays demonstrated that D-C-G3 exhibited a potential novel IgG binding property that was obviously different from those of both parent proteins. This study provides an example of successful protein engineering through in vitro molecular evolution and useful approaches for structure and function studies of IBPs.
Figures
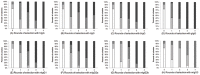
 , phage clones with no inserted fragment;
, phage clones with no inserted fragment;  , phage clones displaying one domain of the combinatorial Ig-binding molecules;
, phage clones displaying one domain of the combinatorial Ig-binding molecules;  , phage clones displaying two domains of the combinatorial Ig-binding molecules;
, phage clones displaying two domains of the combinatorial Ig-binding molecules;  , phage clones displaying three domains of the combinatorial Ig-binding molecules.
, phage clones displaying three domains of the combinatorial Ig-binding molecules.
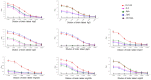
 , D-C-G3;
, D-C-G3; , D-C;
, D-C;  , SpA;
, SpA;  , SpG; -, PET-32A control protein.
, SpG; -, PET-32A control protein.
 , D-C-G3;
, D-C-G3; , D-C;
, D-C;  , SpA;
, SpA;  , SpG; -, PET-32A control protein. The inhibition of the binding of SpA to bIgG, gIgG and mIgG2a was not shown, as the binding activities of SpA were too weak to be inhibited.
, SpG; -, PET-32A control protein. The inhibition of the binding of SpA to bIgG, gIgG and mIgG2a was not shown, as the binding activities of SpA were too weak to be inhibited.
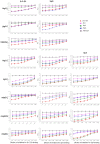
 , No. 142 amino acid residue in mouse immunoglobulin gamma 1 heavy chain.
, No. 142 amino acid residue in mouse immunoglobulin gamma 1 heavy chain.
References
-
- Kronvall G. & Jonsson K. Receptins: a novel term for an expanding spectrum of natural and engineered microbial proteins with binding properties for mammalian proteins. J Mol Recognit 12, 38–44 (1999). - PubMed
-
- Uhlen M. et al. Complete sequence of the staphylococcal gene encoding protein A. A gene evolved through multiple duplications. J Biol Chem 259, 1695–1702 (1984). - PubMed
-
- Moks T. et al. Staphylococcal protein A consists of five IgG-binding domains. Eur J Biochem 156, 637–643 (1986). - PubMed
-
- Gouda H. et al. Three-dimensional solution structure of the B domain of staphylococcal protein A: comparisons of the solution and crystal structures. Biochemistry 31, 9665–9672 (1992). - PubMed
-
- Stone G. C. et al. The Fc binding site for streptococcal protein G is in the C gamma 2-C gamma 3 interface region of IgG and is related to the sites that bind staphylococcal protein A and human rheumatoid factors. J Immunol 143, 565–570 (1989). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

