Temperature sensitivity of two-pore (K2P) potassium channels
- PMID: 25366235
- PMCID: PMC4794111
- DOI: 10.1016/B978-0-12-800181-3.00005-1
Temperature sensitivity of two-pore (K2P) potassium channels
Abstract
At normal body temperature, the two-pore potassium channels TREK-1 (K2P2.1/KCNK2), TREK-2 (K2P10.1/KCNK10), and TRAAK (K2P4.1/KCNK2) regulate cellular excitability by providing voltage-independent leak of potassium. Heat dramatically potentiates K2P channel activity and further affects excitation. This review focuses on the current understanding of the physiological role of heat-activated K2P current, and discusses the molecular mechanism of temperature gating in TREK-1, TREK-2, and TRAAK.
Keywords: Ion channel; K(2P); Potassium channel; TRAAK; TREK-1; TREK-2; Thermosensitivity; Thermotransduction; Two-pore.
Figures

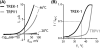
References
-
- Arnadottir J, Chalfie M. Eukaryotic mechanosensitive channels. Annual Review of Biophysics. 2010;39:111–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

