The RtcB RNA ligase is an essential component of the metazoan unfolded protein response
- PMID: 25366321
- PMCID: PMC4264930
- DOI: 10.15252/embr.201439531
The RtcB RNA ligase is an essential component of the metazoan unfolded protein response
Abstract
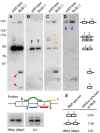
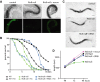
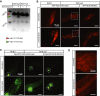
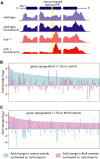
RNA ligation can regulate RNA function by altering RNA sequence, structure and coding potential. For example, the function of XBP1 in mediating the unfolded protein response requires RNA ligation, as does the maturation of some tRNAs. Here, we describe a novel in vivo model in Caenorhabditis elegans for the conserved RNA ligase RtcB and show that RtcB ligates the xbp-1 mRNA during the IRE-1 branch of the unfolded protein response. Without RtcB, protein stress results in the accumulation of unligated xbp-1 mRNA fragments, defects in the unfolded protein response, and decreased lifespan. RtcB also ligates endogenous pre-tRNA halves, and RtcB mutants have defects in growth and lifespan that can be bypassed by expression of pre-spliced tRNAs. In addition, animals that lack RtcB have defects that are independent of tRNA maturation and the unfolded protein response. Thus, RNA ligation by RtcB is required for the function of multiple endogenous target RNAs including both xbp-1 and tRNAs. RtcB is uniquely capable of performing these ligation functions, and RNA ligation by RtcB mediates multiple essential processes in vivo.
Keywords: Caenorhabditis elegans; HSPC117; RtcB; tRNA; xbp‐1.
© 2014 The Authors.
Figures
Comment in
-
Making ends meet: a role of RNA ligase RTCB in unfolded protein response.EMBO J. 2014 Dec 17;33(24):2887-9. doi: 10.15252/embj.201490425. Epub 2014 Nov 17. EMBO J. 2014. PMID: 25404664 Free PMC article.
References
-
- Filipowicz W, Shatkin AJ. Origin of splice junction phosphate in tRNAs processed by HeLa cell extract. Cell. 1983;32:547–557. - PubMed
-
- Laski FA, Fire AZ, RajBhandary UL, Sharp PA. Characterization of tRNA precursor splicing in mammalian extracts. J Biol Chem. 1983;258:11974–11980. - PubMed
-
- Paushkin SV, Patel M, Furia BS, Peltz SW, Trotta CR. Identification of a human endonuclease complex reveals a link between tRNA splicing and pre-mRNA 3′ end formation. Cell. 2004;117:311–321. - PubMed
-
- Shen X, Ellis RE, Lee K, Liu CY, Yang K, Solomon A, Yoshida H, Morimoto R, Kurnit DM, Mori K, et al. Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell. 2001;107:893–903. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32GM008730/GM/NIGMS NIH HHS/United States
- T32 GM007223/GM/NIGMS NIH HHS/United States
- P40 OD010440/OD/NIH HHS/United States
- R01 GM073863/GM/NIGMS NIH HHS/United States
- R01GM073863/GM/NIGMS NIH HHS/United States
- R01 GM048410/GM/NIGMS NIH HHS/United States
- R01 NS066082/NS/NINDS NIH HHS/United States
- P30 CA046934/CA/NCI NIH HHS/United States
- R56 NS081217/NS/NINDS NIH HHS/United States
- T32 GM008730/GM/NIGMS NIH HHS/United States
- R56NS081217/NS/NINDS NIH HHS/United States
- R01GM048410/GM/NIGMS NIH HHS/United States
- T32GM007223/GM/NIGMS NIH HHS/United States
- R01NS066082/NS/NINDS NIH HHS/United States
- P40OD010440/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

