Synthesis of α-amino acid derivatives and peptides via enantioselective addition of masked acyl cyanides to imines
- PMID: 25366558
- PMCID: PMC4244832
- DOI: 10.1021/ja510135t
Synthesis of α-amino acid derivatives and peptides via enantioselective addition of masked acyl cyanides to imines
Abstract
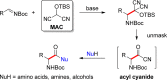
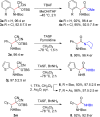
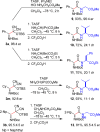
A general, asymmetric synthesis of amino acid derivatives is reported. Masked acyl cyanide (MAC) reagents are shown to be effective umpolung synthons for enantioselective additions to N-Boc-aldimines. The reactions are catalyzed by a modified cinchona alkaloid, which can function as a bifunctional, hydrogen bonding catalyst, and afford adducts in excellent yields (90-98%) and high enantioselectivities (up to 97.5:2.5 er). Unmasking the addition products gives acyl cyanide intermediates that are intercepted by a variety of nucleophiles to afford α-amino acid derivatives. Notably, the methodology provides an alternative method for peptide bond formation.
Figures
References
-
- Soloshonok V. A.; Izawa K.. Asymmetric Synthesis and Application of α-Amino Acids; Oxford University Press: Washington, DC, 2009.
- Blaskovich M. A.Handbook on Syntheses of Amino Acids: General Routes for the Syntheses of Amino Acids; Oxford University Press: Oxford; New York, 2010.
-
- Blaser H. U. Chem. Rev. 1992, 92, 935.
- Wiley R. A.; Rich D. H. Med. Res. Rev. 1993, 13, 327. - PubMed
- Davie E. A. C.; Mennen S. M.; Xu Y. J.; Miller S. J. Chem. Rev. 2007, 107, 5759. - PubMed
- Vlieghe P.; Lisowski V.; Martinez J.; Khrestchatisky M. Drug Discovery Today 2010, 15, 40. - PubMed
- Rabone J.; Yue Y. F.; Chong S. Y.; Stylianou K. C.; Bacsa J.; Bradshaw D.; Darling G. R.; Berry N. G.; Khimyak Y. Z.; Ganin A. Y.; Wiper P.; Claridge J. B.; Rosseinsky M. J. Science 2010, 329, 1053. - PubMed
-
-
For selected examples of recent metal free asymmetric Strecker reactions, see:
- Ooi T.; Uematsu Y.; Maruoka K. J. Am. Chem. Soc. 2006, 128, 2548. - PubMed
- Zuend S. J.; Coughlin M. P.; Lalonde M. P.; Jacobsen E. N. Nature 2009, 461, 968. - PMC - PubMed
- Yan H.; Suk O. J.; Lee J. W.; Song C. E. Nat. Commun. 2012, 3, 1212. - PubMed
-
For metal catalyzed asymmetric Strecker reactions, see:
- Sigman M. S.; Jacobsen E. N. J. Am. Chem. Soc. 1998, 120, 5315.
- Krueger C. A.; Kuntz K. W.; Dzierba C. D.; Wirschun W. G.; Gleason J. D.; Snapper M. L.; Hoveyda A. H. J. Am. Chem. Soc. 1999, 121, 4284.
- Masumoto S.; Usuda H.; Suzuki M.; Kanai M.; Shibasaki M. J. Am. Chem. Soc. 2003, 125, 5634. - PubMed
- Abell J. P.; Yamamoto H. J. Am. Chem. Soc. 2009, 131, 15118. - PubMed
-
-
-
Many researchers have pointed out the sensitivity of Strecker products to epimerization. See:
- Sigman M. S.; Vachal P.; Jacobsen E. N. Angew. Chem., Int. Ed. 2000, 39, 1279. - PubMed
- Porter J. R.; Wirschun W. G.; Kuntz K. W.; Snapper M. L.; Hoveyda A. H. J. Am. Chem. Soc. 2000, 122, 2657.
- Banphavichit V.; Mansawat W.; Bhanthumnavin W.; Vilaivan T. Tetrahedron 2009, 65, 5849.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources