Randomized phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the Radiation Therapy Oncology Group 0129 trial: long-term report of efficacy and toxicity
- PMID: 25366680
- PMCID: PMC4239304
- DOI: 10.1200/JCO.2014.55.3925
Randomized phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the Radiation Therapy Oncology Group 0129 trial: long-term report of efficacy and toxicity
Abstract
Purpose: We tested the efficacy and toxicity of cisplatin plus accelerated fractionation with a concomitant boost (AFX-C) versus standard fractionation (SFX) in locally advanced head and neck carcinoma (LA-HNC).
Patients and methods: Patients had stage III to IV carcinoma of the oral cavity, oropharynx, hypopharynx, or larynx. Radiation therapy schedules were 70 Gy in 35 fractions over 7 weeks (SFX) or 72 Gy in 42 fractions over 6 weeks (AFX-C). Cisplatin doses were 100 mg/m(2) once every 3 weeks for two (AFX-C) or three (SFX) cycles. Toxicities were scored by using National Cancer Institute Common Toxicity Criteria 2.0 and the Radiation Therapy Oncology Group/European Organisation for Research and Treatment of Cancer criteria. Overall survival (OS) and progression-free survival (PFS) rates were estimated by using the Kaplan-Meier method and were compared by using the one-sided log-rank test. Locoregional failure (LRF) and distant metastasis (DM) rates were estimated by using the cumulative incidence method and Gray's test.
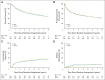
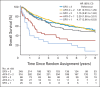
Results: In all, 721 of 743 patients were analyzable (361, SFX; 360, AFX-C). At a median follow-up of 7.9 years (range, 0.3 to 10.1 years) for 355 surviving patients, no differences were observed in OS (hazard ratio [HR], 0.96; 95% CI, 0.79 to 1.18; P = .37; 8-year survival, 48% v 48%), PFS (HR, 1.02; 95% CI, 0.84 to 1.24; P = .52; 8-year estimate, 42% v 41%), LRF (HR, 1.08; 95% CI, 0.84 to 1.38; P = .78; 8-year estimate, 37% v 39%), or DM (HR, 0.83; 95% CI, 0.56 to 1.24; P = .16; 8-year estimate, 15% v 13%). For oropharyngeal cancer, p16-positive patients had better OS than p16-negative patients (HR, 0.30; 95% CI, 0.21 to 0.42; P < .001; 8-year survival, 70.9% v 30.2%). There were no statistically significant differences in the grade 3 to 5 acute or late toxicities between the two arms and p-16 status.
Conclusion: When combined with cisplatin, AFX-C neither improved outcome nor increased late toxicity in patients with LA-HNC. Long-term high survival rates in p16-positive patients with oropharyngeal cancer support the ongoing efforts to explore deintensification.
Trial registration: ClinicalTrials.gov NCT00047008.
© 2014 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
[Accelerated versus conventional fractionation in combined chemoradiotherapy of head and neck tumors].Strahlenther Onkol. 2015 Apr;191(4):384-6. doi: 10.1007/s00066-015-0819-1. Strahlenther Onkol. 2015. PMID: 26079027 German. No abstract available.
References
-
- Bourhis J, Overgaard J, Audry H, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: A meta-analysis. Lancet. 2006;368:843–854. - PubMed
-
- Pignon JP, Bourhis J, Domenge C, et al. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: Three meta-analyses of updated individual data—MACH-NC Collaborative Group: Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet. 2000;355:949–955. - PubMed
-
- Pignon JP, le Maître A, Maillard E, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. - PubMed
-
- Fu KK, Pajak TF, Trotti A, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: First report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000;48:7–16. - PubMed
-
- Ang KK, Harris J, Garden AS, et al. Concomitant boost radiation plus concurrent cisplatin for advanced head and neck carcinomas: Radiation Therapy Oncology Group phase II trial 99-14. J Clin Oncol. 2005;23:3008–3015. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

