Biocompatibility of tungsten disulfide inorganic nanotubes and fullerene-like nanoparticles with salivary gland cells
- PMID: 25366879
- PMCID: PMC4356479
- DOI: 10.1089/ten.TEA.2014.0163
Biocompatibility of tungsten disulfide inorganic nanotubes and fullerene-like nanoparticles with salivary gland cells
Abstract
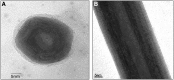
Impaired salivary gland (SG) function leading to oral diseases is relatively common with no adequate solution. Previously, tissue engineering of SG had been proposed to overcome this morbidity, however, not yet clinically available. Multiwall inorganic (tungsten disulfide [WS2]) nanotubes (INT-WS2) and fullerene-like nanoparticles (IF-WS2) have many potential medical applications. A yet unexplored venue application is their interaction with SG, and therefore, our aim was to test the biocompatibility of INT/IF-WS2 with the A5 and rat submandibular cells (RSC) SG cells. The cells were cultured and subjected after 1 day to different concentrations of INT-WS2 and were compared to control groups. Growth curves, trypan blue viability test, and carboxyfluorescein succinimidyl ester (CFSE) proliferation assay were obtained. Furthermore, cells morphology and interaction with the nanoparticles were observed by light microscopy, scanning electron microscopy and transmission electron microscopy (TEM), and energy dispersive X-ray spectroscopy. The results showed no significant differences in growth curves, proliferation kinetics, and viability between the groups compared. Moreover, no alterations were observed in the cell morphology. Interestingly, TEM images indicated that the nanoparticles are uptaken by the cells and accumulate in cytoplasmic vesicles. These results suggest promising future medical applications for these nanoparticles.
Figures








Similar articles
-
Low cytotoxicity of inorganic nanotubes and fullerene-like nanostructures in human bronchial epithelial cells: relation to inflammatory gene induction and antioxidant response.Environ Sci Technol. 2014 Mar 18;48(6):3457-66. doi: 10.1021/es500065z. Epub 2014 Feb 26. Environ Sci Technol. 2014. PMID: 24533583
-
Recent progress in the research of inorganic fullerene-like nanoparticles and inorganic nanotubes.Chem Soc Rev. 2010 May;39(5):1423-34. doi: 10.1039/b901466g. Epub 2009 Dec 3. Chem Soc Rev. 2010. PMID: 20419198 Review.
-
Encapsulation of Mo₂C in MoS₂ inorganic fullerene-like nanoparticles and nanotubes.Nanoscale. 2013 Feb 21;5(4):1499-502. doi: 10.1039/c2nr33828a. Nanoscale. 2013. PMID: 23338052
-
Inorganic fullerene-like tungsten disulfide nanocoating for friction reduction of nickel-titanium alloys.Nanomedicine (Lond). 2009 Dec;4(8):943-50. doi: 10.2217/nnm.09.68. Nanomedicine (Lond). 2009. PMID: 19958230
-
Inorganic nanotubes and fullerene-like nanoparticles.Nat Nanotechnol. 2006 Nov;1(2):103-11. doi: 10.1038/nnano.2006.62. Nat Nanotechnol. 2006. PMID: 18654160 Review.
Cited by
-
Unleashing the potential of tungsten disulfide: Current trends in biosensing and nanomedicine applications.Heliyon. 2024 Jan 11;10(2):e24427. doi: 10.1016/j.heliyon.2024.e24427. eCollection 2024 Jan 30. Heliyon. 2024. PMID: 38293340 Free PMC article. Review.
-
SPIO-Au core-shell nanoparticles for promoting osteogenic differentiation of MC3T3-E1 cells: Concentration-dependence study.J Biomed Mater Res A. 2017 Dec;105(12):3350-3359. doi: 10.1002/jbm.a.36200. Epub 2017 Sep 19. J Biomed Mater Res A. 2017. PMID: 28869707 Free PMC article.
-
Development of Biocompatible Polyhydroxyalkanoate/Chitosan-Tungsten Disulphide Nanocomposite for Antibacterial and Biological Applications.Polymers (Basel). 2022 May 30;14(11):2224. doi: 10.3390/polym14112224. Polymers (Basel). 2022. PMID: 35683897 Free PMC article.
-
Short Pulse Laser Synthesis of Transition-Metal Dichalcogenide Nanostructures under Ambient Conditions.ACS Omega. 2017 Jun 14;2(6):2649-2656. doi: 10.1021/acsomega.7b00409. eCollection 2017 Jun 30. ACS Omega. 2017. PMID: 31457606 Free PMC article.
-
Effect of WS₂ Inorganic Nanotubes on Isothermal Crystallization Behavior and Kinetics of Poly(3-Hydroxybutyrate-co-3-hydroxyvalerate).Polymers (Basel). 2018 Feb 9;10(2):166. doi: 10.3390/polym10020166. Polymers (Basel). 2018. PMID: 30966202 Free PMC article.
References
-
- Margulis L., Salitra G., Tenne R., and Talianker M.Nested fullerene-like structures. Nature 365,113, 1993 - PubMed
-
- Tenne R., Margulis L., Genut M., and Hodes G.Polyhedral and cylindrical structures of tungsten disulfide. Nature 360,444, 1992
-
- Tenne R.Inorganic nanotubes and fullerene-like nanoparticles. Nat Nanotechnol 1,103, 2006 - PubMed
-
- Rapoport L., Bilik Y., Feldman Y., Homyonfer M., Cohen S.R., and Tenne R.Hollow nanoparticles of WS2 as potential solid-state lubricants. Nature 387,791, 1997
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

