The clinical utility window for acute kidney injury biomarkers in the critically ill
- PMID: 25366893
- PMCID: PMC4255650
- DOI: 10.1186/s13054-014-0601-2
The clinical utility window for acute kidney injury biomarkers in the critically ill
Abstract
Introduction: Acute Kidney Injury (AKI) biomarker utility depends on sample timing after the onset of renal injury. We compared biomarker performance on arrival in the emergency department (ED) with subsequent performance in the intensive care unit (ICU).
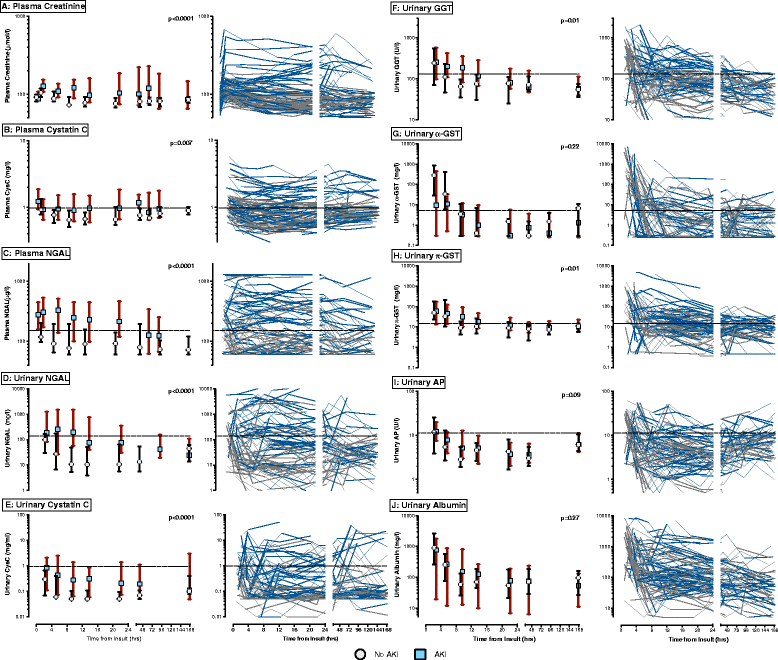
Methods: Urinary and plasma Neutrophil Gelatinase-Associated Lipocalin (NGAL), and urinary Cystatin C (CysC), alkaline phosphatase, γ-Glutamyl Transpeptidase (GGT), α- and π-Glutathione S-Transferase (GST), and albumin were measured on ED presentation, and at 0, 4, 8, and 16 hours, and days 2, 4 and 7 in the ICU in patients after cardiac arrest, sustained or profound hypotension or ruptured abdominal aortic aneurysm. AKI was defined as plasma creatinine increase ≥ 26.5 μmol/l within 48 hours or ≥ 50% within 7 days.
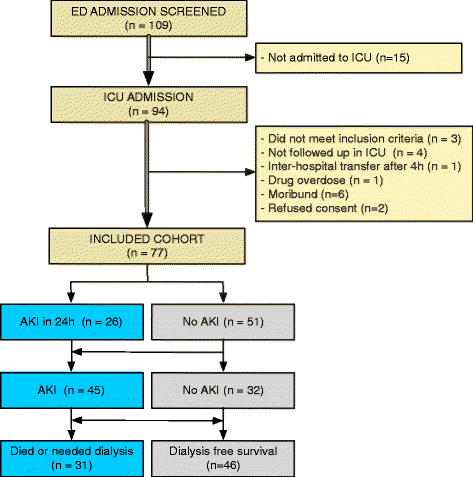
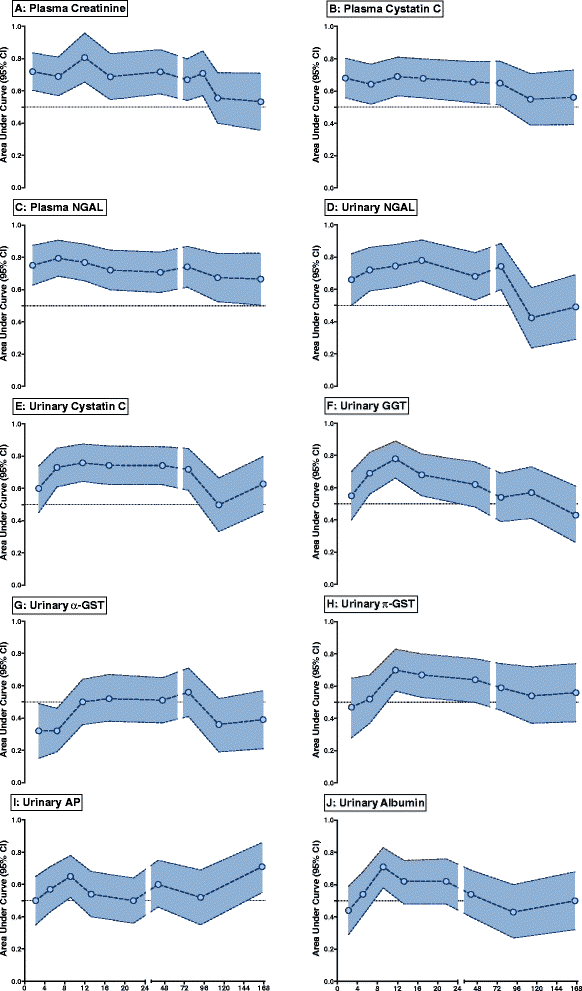
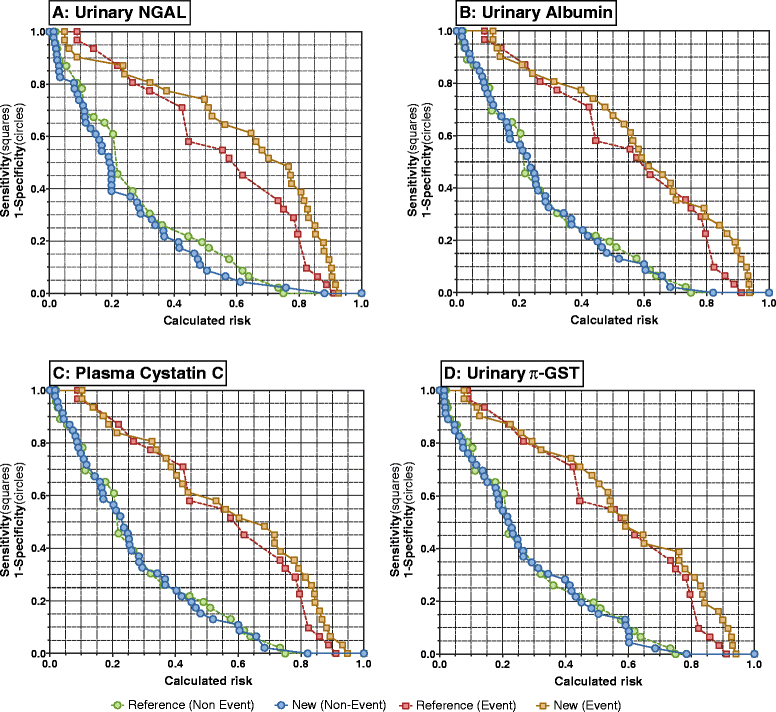
Results: In total, 45 of 77 patients developed AKI. Most AKI patients had elevated urinary NGAL, and plasma NGAL and CysC in the period 6 to 24 hours post presentation. Biomarker performance in the ICU was similar or better than when measured earlier in the ED. Plasma NGAL diagnosed AKI at all sampling times, urinary NGAL, plasma and urinary CysC up to 48 hours, GGT 4 to 12 hours, and π-GST 8 to 12 hours post insult. Thirty-one patients died or required dialysis. Peak 24-hour urinary NGAL and albumin independently predicted 30-day mortality and dialysis; odds ratios 2.87 (1.32 to 6.26), and 2.72 (1.14 to 6.48), respectively. Urinary NGAL improved risk prediction by 11% (IDI event of 0.06 (0.002 to 0.19) and IDI non-event of 0.04 (0.002 to 0.12)).
Conclusion: Early measurement in the ED has utility, but not better AKI diagnostic performance than later ICU measurement. Plasma NGAL diagnosed AKI at all time points. Urinary NGAL best predicted mortality or dialysis compared to other biomarkers.
Trial registration: Australian and New Zealand Clinical Trials Registry ACTRN12610001012066. Registered 12 February 2010.
Figures
Similar articles
-
Plasma and urine neutrophil gelatinase-associated lipocalin in the diagnosis of new onset acute kidney injury in critically ill patients.Crit Care. 2014 Jul 1;18(4):R137. doi: 10.1186/cc13958. Crit Care. 2014. PMID: 24985156 Free PMC article.
-
Impact of severe sepsis on serum and urinary biomarkers of acute kidney injury in critically ill children: an observational study.Blood Purif. 2013;35(1-3):172-6. doi: 10.1159/000346629. Epub 2013 Feb 15. Blood Purif. 2013. PMID: 23428967
-
Rapid detection of acute kidney injury by plasma and urinary neutrophil gelatinase-associated lipocalin after cardiopulmonary bypass.J Cardiovasc Pharmacol. 2009 Mar;53(3):261-6. doi: 10.1097/FJC.0b013e31819d6139. J Cardiovasc Pharmacol. 2009. PMID: 19247188 Clinical Trial.
-
Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for early acute kidney injury.Crit Care Clin. 2011 Apr;27(2):379-89. doi: 10.1016/j.ccc.2010.12.003. Crit Care Clin. 2011. PMID: 21440207 Review.
-
Clinical review: Predictive value of neutrophil gelatinase-associated lipocalin for acute kidney injury in intensive care patients.Crit Care. 2013 Apr 24;17(2):211. doi: 10.1186/cc11855. Crit Care. 2013. PMID: 23680259 Free PMC article.
Cited by
-
Subclinical acute kidney injury is associated with adverse outcomes in critically ill neonates and children.Crit Care. 2018 Oct 10;22(1):256. doi: 10.1186/s13054-018-2193-8. Crit Care. 2018. PMID: 30305134 Free PMC article.
-
Biomarker-Guided Risk Assessment for Acute Kidney Injury: Time for Clinical Implementation?Ann Lab Med. 2021 Jan;41(1):1-15. doi: 10.3343/alm.2021.41.1.1. Epub 2020 Aug 25. Ann Lab Med. 2021. PMID: 32829575 Free PMC article. Review.
-
Urinary π-glutathione S-transferase Predicts Advanced Acute Kidney Injury Following Cardiovascular Surgery.Sci Rep. 2016 Aug 16;6:26335. doi: 10.1038/srep26335. Sci Rep. 2016. PMID: 27527370 Free PMC article.
-
Evaluation of clinically available renal biomarkers in critically ill adults: a prospective multicenter observational study.Crit Care. 2017 Mar 7;21(1):46. doi: 10.1186/s13054-017-1626-0. Crit Care. 2017. PMID: 28264714 Free PMC article.
-
Prognostic Biomarkers and AKI: Potential to Enhance the Identification of Post-Operative Patients at Risk of Loss of Renal Function.Res Rep Urol. 2024 Mar 5;16:65-78. doi: 10.2147/RRU.S385856. eCollection 2024. Res Rep Urol. 2024. PMID: 38476861 Free PMC article. Review.
References
-
- Endre ZH, Pickering JW, Walker RJ, Devarajan P, Edelstein CL, Bonventre JV, Frampton CM, Bennett MR, Ma Q, Sabbisetti VS, Vaidya VS, Walcher AM, Shaw GM, Henderson SJ, Nejat M, Schollum JBW, George PM. Improved performance of urinary biomarkers of acute kidney injury in the critically ill by stratification for injury duration and baseline renal function. Kidney Int. 2011;79:1119–1130. doi: 10.1038/ki.2010.555. - DOI - PMC - PubMed
-
- Endre ZH, Walker RJ, Pickering JW, Shaw GM, Frampton CM, Henderson SJ, Hutchison R, Mehrtens JE, Robinson JM, Schollum JBW, Westhuyzen J, Celi LA, McGinley RJ, Campbell IJ, George PM. Early intervention with erythropoietin does not affect the outcome of acute kidney injury (the EARLYARF trial) Kidney Int. 2010;77:1020–1030. doi: 10.1038/ki.2010.25. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

