Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation
- PMID: 25366965
- PMCID: PMC4235643
- DOI: 10.1084/jem.20140625
Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation
Erratum in
- J Exp Med. 2014 Dec 15;211(13):2683
- J Exp Med. 2014 Nov 17;211(12):2396-7
Abstract
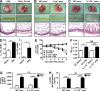
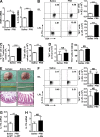
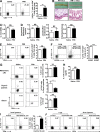
Influenza in humans is often accompanied by gastroenteritis-like symptoms such as diarrhea, but the underlying mechanism is not yet understood. We explored the occurrence of gastroenteritis-like symptoms using a mouse model of respiratory influenza infection. We found that respiratory influenza infection caused intestinal injury when lung injury occurred, which was not due to direct intestinal viral infection. Influenza infection altered the intestinal microbiota composition, which was mediated by IFN-γ produced by lung-derived CCR9(+)CD4(+) T cells recruited into the small intestine. Th17 cells markedly increased in the small intestine after PR8 infection, and neutralizing IL-17A reduced intestinal injury. Moreover, antibiotic depletion of intestinal microbiota reduced IL-17A production and attenuated influenza-caused intestinal injury. Further study showed that the alteration of intestinal microbiota significantly stimulated IL-15 production from intestinal epithelial cells, which subsequently promoted Th17 cell polarization in the small intestine in situ. Thus, our findings provide new insights into an undescribed mechanism by which respiratory influenza infection causes intestinal disease.
© 2014 Wang et al.
Figures





Comment in
-
Mucosal immunology: sick of the flu.Nat Rev Immunol. 2014 Dec;14(12):781. doi: 10.1038/nri3773. Epub 2014 Nov 14. Nat Rev Immunol. 2014. PMID: 25394944 No abstract available.
-
FLUshing in the bathroom.J Exp Med. 2014 Nov 17;211(12):2328-9. doi: 10.1084/jem.21112insight4. J Exp Med. 2014. PMID: 25403806 Free PMC article. No abstract available.
Similar articles
-
CCR9+ T cells contribute to the resolution of the inflammatory response in a mouse model of intestinal amoebiasis.Immunobiology. 2012 Aug;217(8):795-807. doi: 10.1016/j.imbio.2012.04.005. Epub 2012 May 4. Immunobiology. 2012. PMID: 22633147
-
Mucosal immunology: sick of the flu.Nat Rev Immunol. 2014 Dec;14(12):781. doi: 10.1038/nri3773. Epub 2014 Nov 14. Nat Rev Immunol. 2014. PMID: 25394944 No abstract available.
-
The imbalance of pulmonary Th17/Treg cells in BALB/c suckling mice infected with respiratory syncytial virus-mediated intestinal immune damage and gut microbiota changes.Microbiol Spectr. 2024 Jun 4;12(6):e0328323. doi: 10.1128/spectrum.03283-23. Epub 2024 May 10. Microbiol Spectr. 2024. PMID: 38727214 Free PMC article.
-
Pouring fuel on the fire: Th17 cells, the environment, and autoimmunity.J Clin Invest. 2015 Jun;125(6):2211-9. doi: 10.1172/JCI78085. Epub 2015 May 11. J Clin Invest. 2015. PMID: 25961452 Free PMC article. Review.
-
Microbiota-specific Th17 Cells: Yin and Yang in Regulation of Inflammatory Bowel Disease.Inflamm Bowel Dis. 2016 Jun;22(6):1473-82. doi: 10.1097/MIB.0000000000000775. Inflamm Bowel Dis. 2016. PMID: 27057688 Free PMC article. Review.
Cited by
-
Abscopal effect: from a rare phenomenon to a new frontier in cancer therapy.Biomark Res. 2024 Sep 4;12(1):98. doi: 10.1186/s40364-024-00628-3. Biomark Res. 2024. PMID: 39228005 Free PMC article. Review.
-
Anti-influenza A virus activity of two Newtonia species and the isolated compound myricetin-3-o-rhamnoside.BMC Complement Med Ther. 2021 Mar 16;21(1):92. doi: 10.1186/s12906-021-03250-0. BMC Complement Med Ther. 2021. PMID: 33726731 Free PMC article.
-
Short-Chain Fatty Acids as a Potential Treatment for Infections: a Closer Look at the Lungs.Infect Immun. 2021 Aug 16;89(9):e0018821. doi: 10.1128/IAI.00188-21. Epub 2021 Aug 16. Infect Immun. 2021. PMID: 34097474 Free PMC article. Review.
-
The 16S rRNA Gene Sequencing of Gut Microbiota in Chickens Infected with Different Virulent Newcastle Disease Virus Strains.Animals (Basel). 2022 Sep 24;12(19):2558. doi: 10.3390/ani12192558. Animals (Basel). 2022. PMID: 36230299 Free PMC article.
-
Potential Causes and Consequences of Gastrointestinal Disorders during a SARS-CoV-2 Infection.Cell Rep. 2020 Jul 21;32(3):107915. doi: 10.1016/j.celrep.2020.107915. Epub 2020 Jul 3. Cell Rep. 2020. PMID: 32649864 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

