Post-stroke fatigue: a deficit in corticomotor excitability?
- PMID: 25367024
- PMCID: PMC4441078
- DOI: 10.1093/brain/awu306
Post-stroke fatigue: a deficit in corticomotor excitability?
Abstract
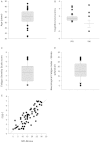
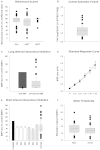
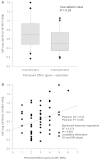
The pathophysiology of post-stroke fatigue is poorly understood although it is thought to be a consequence of central nervous system pathophysiology. In this study we investigate the relationship between corticomotor excitability and self-reported non-exercise related fatigue in chronic stroke population. Seventy first-time non-depressed stroke survivors (60.36 ± 12.4 years, 20 females, 56.81 ± 63 months post-stroke) with minimal motor and cognitive impairment were included in the cross-sectional observational study. Fatigue was measured using two validated questionnaires: Fatigue Severity Scale 7 and Neurological Fatigue Index - Stroke. Perception of effort was measured using a 0-10 numerical rating scale in an isometric biceps hold-task and was used as a secondary measure of fatigue. Neurophysiological measures of corticomotor excitability were performed using transcranial magnetic stimulation. Corticospinal excitability was quantified using resting and active motor thresholds and stimulus-response curves of the first dorsal interosseous muscle. Intracortical M1 excitability was measured using paired pulse paradigms: short and long interval intracortical inhibition in the same hand muscle as above. Excitability of cortical and subcortical inputs that drive M1 output was measured in the biceps muscle using a modified twitch interpolation technique to provide an index of central activation failure. Stepwise regression was performed to determine the explanatory variables that significantly accounted for variance in the fatigue and perception scores. Resting motor threshold (R = 0.384; 95% confidence interval = 0.071; P = 0.036) accounted for 14.7% (R(2)) of the variation in Fatigue Severity Scale 7. Central activation failure (R = 0.416; 95% confidence interval = -1.618; P = 0.003) accounted for 17.3% (R(2)) of the variation in perceived effort score. Thus chronic stroke survivors with high fatigue exhibit high motor thresholds and those who perceive high effort have low excitability of inputs that drive motor cortex output. We suggest that low excitability of both corticospinal output and its facilitatory synaptic inputs from cortical and sub-cortical sites contribute to high levels of fatigue after stroke.
Keywords: behavioural neurology; motor cortex; motor evoked potentials; stroke rehabilitation; transcranial magnetic stimulation.
© The Author (2014). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures




Similar articles
-
Influence of post-stroke fatigue on reaction times and corticospinal excitability during movement preparation.Clin Neurophysiol. 2021 Jan;132(1):191-199. doi: 10.1016/j.clinph.2020.11.012. Epub 2020 Nov 25. Clin Neurophysiol. 2021. PMID: 33302061 Free PMC article.
-
Neural effective connectivity explains subjective fatigue in stroke.Brain. 2022 Mar 29;145(1):285-294. doi: 10.1093/brain/awab287. Brain. 2022. PMID: 34791073 Free PMC article.
-
Primary Motor Cortex Excitability During Recovery After Stroke: Implications for Neuromodulation.Brain Stimul. 2015 Nov-Dec;8(6):1183-90. doi: 10.1016/j.brs.2015.06.015. Epub 2015 Jun 30. Brain Stimul. 2015. PMID: 26195321
-
Stimulation of the motor cortex and corticospinal tract to assess human muscle fatigue.Neuroscience. 2013 Feb 12;231:384-99. doi: 10.1016/j.neuroscience.2012.10.058. Epub 2012 Nov 3. Neuroscience. 2013. PMID: 23131709 Review.
-
Corticomotor pathway function and recovery after stroke: a look back and a way forward.J Physiol. 2025 Feb;603(3):651-662. doi: 10.1113/JP285562. Epub 2024 May 30. J Physiol. 2025. PMID: 38814805 Free PMC article. Review.
Cited by
-
Effort, success, and side of lesion determine arm choice in individuals with chronic stroke.J Neurophysiol. 2022 Jan 1;127(1):255-266. doi: 10.1152/jn.00532.2020. Epub 2021 Dec 8. J Neurophysiol. 2022. PMID: 34879206 Free PMC article.
-
Electroencephalography-Derived Functional Connectivity in Sensorimotor Networks in Post Stroke Fatigue.Brain Topogr. 2023 Sep;36(5):727-735. doi: 10.1007/s10548-023-00975-8. Epub 2023 Jun 17. Brain Topogr. 2023. PMID: 37328707 Free PMC article.
-
Altered motor cortex physiology and dysexecutive syndrome in patients with fatigue and cognitive difficulties after mild COVID-19.Eur J Neurol. 2022 Jun;29(6):1652-1662. doi: 10.1111/ene.15278. Epub 2022 Feb 24. Eur J Neurol. 2022. PMID: 35138693 Free PMC article.
-
Modafinil treatment modulates functional connectivity in stroke survivors with severe fatigue.Sci Rep. 2019 Jul 4;9(1):9660. doi: 10.1038/s41598-019-46149-0. Sci Rep. 2019. PMID: 31273283 Free PMC article. Clinical Trial.
-
Influence of post-stroke fatigue on reaction times and corticospinal excitability during movement preparation.Clin Neurophysiol. 2021 Jan;132(1):191-199. doi: 10.1016/j.clinph.2020.11.012. Epub 2020 Nov 25. Clin Neurophysiol. 2021. PMID: 33302061 Free PMC article.
References
-
- Andersen G, Christensen D, Kirkevold M, Johnsen SP. Post-stroke fatigue and return to work: a 2-year follow-up. Acta Neurol Scand. 2012;125:248–53. - PubMed
-
- Annoni J-M, Staub F, Bogousslavsky J, Brioschi A. Frequency, characterisation and therapies of fatigue after stroke. Neurol Sci. 2008;29(Suppl 2):S244–6. - PubMed
-
- Boroojerdi B, Battaglia F, Muellbacher W, Cohen LG. Mechanisms influencing stimulus-response properties of the human corticospinal system. Clin Neurophysiol. 2001;112:931–7. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

