Pericellular proteolysis in cancer
- PMID: 25367033
- PMCID: PMC4215179
- DOI: 10.1101/gad.250647.114
Pericellular proteolysis in cancer
Abstract
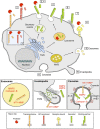
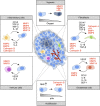
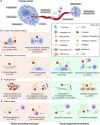
Pericellular proteases have long been associated with cancer invasion and metastasis due to their ability to degrade extracellular matrix components. Recent studies demonstrate that proteases also modulate tumor progression and metastasis through highly regulated and complex processes involving cleavage, processing, or shedding of cell adhesion molecules, growth factors, cytokines, and kinases. In this review, we address how cancer cells, together with their surrounding microenvironment, regulate pericellular proteolysis. We dissect the multitude of mechanisms by which pericellular proteases contribute to cancer progression and discuss how this knowledge can be integrated into therapeutic opportunities.
Keywords: invasion; macrophages; metastasis; migration; proteases; tumor microenvironment.
© 2014 Sevenich and Joyce; Published by Cold Spring Harbor Laboratory Press.
Figures
References
-
- Affara NI, Andreu P, Coussens LM. 2009. Delineating protease functions during cancer development. Methods Mol Biol 539: 1–32 - PubMed
-
- Akkari L, Gocheva V, Kester JC, Hunter KE, Quick ML, Sevenich L, Wang H-W, Peters C, Tang LH, Klimstra D, et al. . 2014. Distinct functions of macrophage- and cancer cell-derived cathepsin Z combine to promote tumor malignancy via interactions with the extracellular matrix. Genes Dev 28: 2134–2150 - PMC - PubMed
-
- Almeida PC, Nantes IL, Rizzi CC, Judice WA, Chagas JR, Juliano L, Nader HB, Tersariol IL. 1999. Cysteine proteinase activity regulation: a possible role of heparin and heparin-like glycosaminoglycans. J Biol Chem 274: 30433–30438 - PubMed
-
- Almeida PC, Nantes IL, Chagas JR, Rizzi CC, Faljoni-Alario A, Carmona E, Juliano L, Nader HB, Tersariol IL. 2001. Cathepsin B activity regulation. Heparin-like glycosaminogylcans protect human cathepsin B from alkaline pH-induced inactivation. J Biol Chem 276: 944–951 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
